Abstract
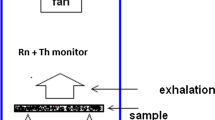
The HO x (OH and HO2) production rate in indoor air caused by radon decay was measured by the chemical amplification technique. The average HO x production rate was found to be (4.31±0.07)×105 HO x per Rn decay per second (Bq) within the range of relative humidity from 3.4 to 55.0 percent at 22 °C. This work providedG(HO x )-value, 7.86±0.13 #/100 eV in air by directly measuring [HO x ] from radiolysis of water vapor. It is also found that there is no obvious relationship between the HO x production rate and the relative humidity in this range. Therefore, this work provides both the basic data for the evaluation of radioactive pollution in indoor air as well as a potentially useful way to produce HO x concentrations in air.
Similar content being viewed by others
References
Atkinson, R., Baulch, D. L., Cox, R. A., Hampson, R. F. Jr., Kerr, J. A., and Troe, J., 1989, Evaluated kinetic and photochemical data for atmospheric chemistry: supplement III,J. Phys. Chem. Ref. Data 18, 881–1097.
Baardsen, E. L. and Terhune, R. W., 1972, Detection of OH in the atmosphere using a dye laser,Appl. Phys. Lett. 21, 209–211.
Campbell, M. J., Sheppard, J. C., and Au, B. F., 1979, Measurement of hydroxyl concentration in boundary layer air by monitoring CO oxidation,Geophys. Res. Lett. 6, 175–178.
Campbell, M. J., Farmer, J. C., Fitzer, C. A., and Henry, M. N., 1986, Radiocarbon tracer measurements of atmospheric hydroxyl radical concentrations,J. Atmos. Chem. 4, 413–427.
Cantrell, C. A. and Stedman, D. H., 1982, A possible technique for the measurement of atmospheric peroxy radicals,Geophys. Res. Lett. 9, 846–849.
Cantrell, C. A., Stedman, D. H., and Wendel, G. J., 1984, Measurement of atmospheric peroxyl radicals by chemical amplification,Anal. Chem. 56, 1496–1502.
Chan, C. Y., O'Brlen, R. J., Hard, T. M., and Cook, T. B., 1983, Laser-excited fluorescence of the hydroxyl radical: relaxation coefficients at atmospheric pressure,J. Phys. Chem. 87, 4966–4974.
Davis, D. D., Heaps, W., and McGee, T., 1976, Direct measurements of natural tropospheric levels of OH via an aircraft borne tunable dye laser,Geophys. Res. Lett. 3, 331–333.
Davis, L. I. Jr., Guo, C., James, J. V., Morris, P. T., Postiff, R., and Wang, C. C., 1985, An airborne lidar instrument for detection of OH using the technique of laser-induced fluoresence,J. Geophys. Res. 90, (D7), 12835–12842.
Davis, L. I. Jr., James, J. V., Wang, C. C., Guo, C., Morris, P. T. and Fishman, J., 1987, OH measurement near the intertropical convergence zone in the Pacific,J. Geophys. Res. 92 (D2), 2020–2024.
Dorn, H.-P., Callies, J., Platt, U., and Ehhalt, D. H., 1988, Measurement of tropospheric OH concentrations by laser long-path absorption spectroscopy,Tellus 40B, 437–445.
Ehhalt, D. H., Dorn, H.-P., and Poppe, D., 1991, The chemistry of the hydroxyl radical in the troposphere,Proc. Royal Soc. Edinburgh 97B, 17–34.
Eisele, F. L. and Tanner, D. J., 1991, Ion-assisted tropospheric OH measurements,J. Geophys. Res. 96 (D5), 9295–9308.
Environmental Protection Agency (EPA), 1992, Technical Support Document for 1992 Citizen's Guide to Radon, Report No. EPA 400-R-92-001, U.S. Environmental Protection Agency, Washington, D.C.
Felton, C. C., Sheppard, J. C., and Campbell, M. J., 1988, Measurements of the diurnal OH cycle by a14C-tracer method,Nature 335, 53–55.
Felton, C. C., Sheppard, J. C., and Campbell, M. J., 1990, The radiochemical hydroxyl radical measurement method,Environ. Sci. Technol. 24, 1841–1847.
Felton, C. C., Sheppard, J. C., and Campbell, M. J., 1992, Precision of the radiochemical OH measurement method,Atmos. Environ. 26A, 2105–2109.
Fishman, J. and Kowalczyk, M., 1980, A brief overview of tropospheric chemistry, inThe CHON Photochemistry of the Troposphere, a colloquium. Nat. Centre Atmos. Res., Boulder, Col.
Goldstein, S. D. and Hopke, P. K., 1985, Environmental neutralization of polonium-218,Environ. Sci. Technol. 19, 146–150.
Hard, T. M., O'Brlen, R. J., Chan, C. Y., and Mehrabzadeh, A. A., 1984, Tropospheric free radical determination by FAGE,Environ. Sci. Technol. 18, 768–777.
Hard, T. M., Chan, C. Y., Mehrabzadeh, A. A., Pan, W. H., and O'Brien, R. J., 1986, Diurnal cycle of tropospheric OH,Nature 322, 617–620.
Hastie, D. R., Weissenmayer, M., Burrows, J. P., and Harris, G. W., 1991, Calibrated chemical amplifier for atmospheric RO x measurements,Anal. Chem. 63, 2048–2057.
Heicklen, J., Westburg, C., and Cohen, N., 1969, Chemical Reactions in Urban Atmospheres (C. S. Tuesday, ed.) Symposium at General Motors Laboratories, Dearborn, MI, 55–58.
Hofzumahaus, A., Dorn, H.-P., Callies, J., Platt, U., and Ehhalt, D. H., 1991, Tropospheric OH concentration measurements by laser long-path absorption spectroscopy,Atmos. Environ. 25A, 2017–2022.
Hubler, G., Perner, D., Platt, U., Tonnissen, A., and Ehhalt, D. H., 1984, Groundlevel OH radical concentration: new measurements by optical absorption,J. Geophys. Res. 89 (D1), 1309–1319.
Hynes, A. J., Wine, P. H., and Semmes, D. H., 1986, Kinetics and mechanism of OH reactions with organic sulfides,J. Phys. Chem. 90, 4148–4156.
Lind, S. C., 1961,Radiation Chemistry of Gases, Reinhold, New York.
Maeda, T., Aoki, K., and Munemori, M., 1980, Chemiluminescence method for the determination of nitrogen dioxide,Anal. Chem. 52, 307–311.
Mätzing, H., 1991, Chemical kinetics of flue gas cleaning by irradiation with electrons,Adv. Chem. Phys. 80, 315–402.
Perner, D., Ehhalt, D. H., Paetz, H. W., Platt, U., Roth, E. P., and Volz, A., 1976, OH radicals in the lower troposphere,Geophys. Res. Lett. 3, 466–468.
Pitts, J. N. Jr., Winer, A. M., Aschmann, S. M., Carter, W. P. L., and Atkinson, R., 1982, Experimental protocol for determining hydroxyl radical reaction rate constants, U.S. EPA project summary.
Platt, U., Rateike, M., Junkermann, W., Hofzumahaus, A., and Ehhalt, D. H., 1987, Detection of atmospheric OH radicals,Free Rad. Res. Comm. 3, 165–172.
Platt, U., Rateike, M., Junkermann, W., Rudolph, J., and Ehhalt, D. H., 1988, New tropospheric OH measurements,J. Geophys. Res. 93 (D5), 5159–5166.
Ramamurthi, M., Strydom, R., Hopke, P. K., and Holub, R. F., 1993, Nanometer and ultrafine aerosols from radon radiolysis,J. Aerosol Sci. 24, 393–407.
Rodgers, M. O., Bradshaw, J. D., Sandholm, S. T., KeSheng, S., and Davis, D. D., 1985, A 2-λ laser-induced fluorescence field instrument for ground-based and airborne measurements of atmospheric OH,J. Geophys. Res. 90(D7), 12819–12834.
Shirinzadeh, B., Wang, C. C., and Deng, D. Q., 1987, Diurnal variation of the OH concentration in ambient air,Geophys. Res. Lett. 14, 123–126.
Wang, C. C. and Davis, L. I. Jr., 1974, Measurement of hydroxyl concentrations in air using a tunable UV laser beam,Phys. Rev. Lett. 32, 349–352.
Wang, C. C., Davis, L. I. Jr., and Selzer, P. M., 1981, Improved airborne measurements of OH in the atmosphere using the technique of laser-induced fluorescences,J. Geophys. Res. 86 (C2), 1181–1186.
Ward, D. C. and Borak, T. B., 1991, Determination of time-varying222Rn concentrations using flow-through scintillation flasks,Health Phys. 61, 799–807.
Weinstock, B., 1969, Carbon monoxide: residence time in the atmosphere,Science 166, 224–225.
Wendel, G. J., Stedman, D. H., and Cantrell, C. A., 1983, Luminol-based nitrogen dioxide detector,Anal. Chem. 55, 937–940.
Willis, G. and Boyd, A. W., 1976, Excitation in the radiation chemistry of inorganic gases,Int. J. Radiat. Phys. Chem. 8, 71–111.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Ding, H., Hopke, P.K. HO x production due to radon decay in air. J Atmos Chem 17, 375–390 (1993). https://doi.org/10.1007/BF00696855
Received:
Revised:
Issue Date:
DOI: https://doi.org/10.1007/BF00696855