Summary
Mummichogs from salt marshes of the Atlantic coast of Maine (USA) experience a mean seasonal temperature range of −1 °C to +15 °C. However, during summer tidal cycles they may experience rapid temperature changes between 15 °C and 30 °C. Observations of animals in the wild suggest that swimming capability is maintained during acute temperature fluctuations which would substantially impair contractile function in other fishes.
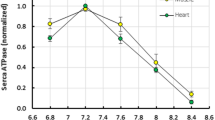
Myofibrils were isolated from the fast epaxial musculature and APTase activity determined in a medium of 40 mM imidazole, pH 7.2 (20 °C), 50 mM KCl, 5 mM ATP, 6 mM MgCl2, 5 mM EGTA, 0.4 mg/ml protein (I=0.124) in the presence (pCa 5.15) and absence (pCa 7.15) of 5 mM CaCl2. More than 90% of the ATPase activity was calcium dependent over the temperature range 0–35 °C. Arrhenius plots of Mg2+ Ca2+ myofibrillar ATPase activity showed a discontinuity in slope at 12.5 °C. Values for activation enthalpy (ΔH≠) of the ATPase were 28,100 cal/mole in the range 0–12.5 °C and 11,900 cal/mole between 12.5–35 °C. The pCa's required to give onehalf maximal ATPase activity were 6.39 (0.407 μM), 6.18 (0.661 μM), and 6.24 (0.575 μM) at 5, 15 and 25 °C, respectively. Thermal denaturation of Ca2+-sensitivity and ATPase activity proceed essentially in parallel. At 0.5 mg/ml in standard incubation medium the temperature required to denature 50% of activity over 30 min is 40–41 °C. Identical results were obtained for groups of fish acclimated for 6 weeks to either 5 °C, 15 °C or 25 °C.
The higher temperature dependence and relatively low ATPase activity at very cold temperatures suggest a relatively torpid state for overwintering fish. Compared to other fishes previously studied, results with mummichogs indicate that both Ca2+ regulatory function and catalytic activity of the myofibrillar complex has low temperature sensitivity over the range of 12–35 °C. These factors apparently obviate the need for acclimatory modifications of the contractile proteins.
Similar content being viewed by others
References
Bárány M (1967) ATPase activity of myosin correlated with speed of muscle shortening. J Gen Physiol 50:197–216
Bendall JR (1969) Muscle, molecules and movement: an essay in the contraction of muscle. American Elsevier, New York
Chidester FE (1920) The behavior ofFundulus heteroclitus in the salt marshes of New Jersey. Am Nat 54:551–557
Fabiato A, Fabiato F (1979) Calculator programs for computing the composition of the solutions containing multiple metals and ligands used for experiments in skinned muscle cells. J Physiol (Paris) 75:463–505
Godt RE, Lindley BD (1982) Influence of temperature upon contractile activation and isometric force production in mechanically skinned muscle fibers of the frog. J Gen Physiol 80:279–297
Hartshorne DJ, Barns EM, Parker L, Fuchs F (1972) The effect of temperature on actomyosin. Biochem Biophys Acta 267:196–202
Itzhaki RF, Gill DM (1964) A microbiuret method for estimating proteins. Anal Biochem 9:401–410
Johnston IA (1979) Calcium regulatory proteins and temperature acclimation of actomyosin ATPase from a eurythermal teleost (Carassius auratus L.). J Comp Physiol 129:163–167
Johnston IA, Davison W, Goldspink G (1975) Adaptations in Mg2+-activated myofibrillar ATPase activity induced by temperature acclimation. FEBS Lett 50:293–295
Johnston IA, Frearson N, Goldspink G (1973) The effects of environmental temperature on the properties of myofibrillar adenosine triphosphatase from various species of fish. Biochem J 133:735–738
Johnston IA, Goldspink G (1975) Thermodynamic activation parameters of fish myofibrillar ATPase enzyme and evolutionary adaptations to temperature. Nature 257:620–622
Johnston IA, Walesby NJ (1977) Molecular mechanisms of temperature adaptation in fish myofibrillar adenosine triphosphatases. J Comp Physiol 119:195–206
Johnston IA, Walesby NJ (1979) Evolutionary temperature adaptation and the calcium regulation of fish actomyosin ATPase. J Comp Physiol 129:169–177
Lehrer GM, Barker R (1970) Conformational changes in rabbit muscle aldolase. Kinetic studies. Biochemistry 9:1533–1539
Lindeman RH, Merenda PF, Gold RZ (1980) Introduction to bivariate and multivariate analysis. Scott, Foresman and Company. Glenview, Illinois, pp 444
Lotrich VA (1975) Summer home range and movements ofFundulus heteroclitus (Pisces: Cyprinodontidae) in a tidal creek. Ecology 56:191–198
Moerland TS, Sidell BD (1981) Characterization of metabolic carbon flow in hepatocytes isolated from thermally acclimated killifish,Fundulus heteroclitus. Physiol Zool 54:379–389
Perrin DD, Sayce IG (1967) Computer calculations of equilibrium concentrations in mixtures of metal ions and complex species. Talanta 14:833–842
Rockstein M, Herron PW (1951) Colorimetric determination of inorganic phosphate in microgram quantities. Anal Chem 23:1500–1501
Sidell BD (1980) Responses of goldfish (Carassius auratus, L.) muscle to acclimation temperature: Alterations in biochemistry and proportions of different fiber types. Physiol Zool 53:98–107
Somero GN (1981) pH-temperature interactions on proteins: principles of optimal pH and buffer system design. Mar Biol Lett 2:163–178
Steel RGD, Torrie JH (1960) Principles and procedures of statistics. McGraw-Hill Book Company, Inc., New York, pp 481
Targett TE (1978) Respiratory metabolism of temperature acclimatedFundulus heteroclitus (L.): zones of compensation and dependence. J Exp Mar Biol Ecol 32:197–206
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Sidell, B.D., Johnston, I.A., Moerland, T.S. et al. The eurythermal myofibrillar protein complex of the mummichog (Fundulus heteroclitus): adaptation to a fluctuating thermal enviroment. J Comp Physiol B 153, 167–173 (1983). https://doi.org/10.1007/BF00689620
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00689620