Summary
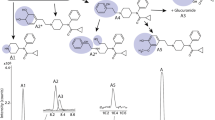
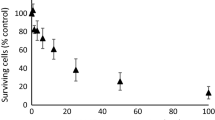
Antineoplastic ether lipids have entered phase I clinical trial and, although their mechanism of action remains unclear, it is widely believed that the plasma membrane is the primary cellular drug target. In the present study the hypothesis was tested that metabolism of ether lipids acts as a detoxification process. [31P]-nuclear magnetic resonance (NMR) spectroscopy was used to study the metabolism of the ether lipid SRI 62-834 (SRI) and the phosphate ester hexadecylphosphocholine (HPC) in the presence of both isolated phospholipases C and D and post-mitochondrial rat liver homogenate. Both SRI and HPC were slowly metabolised by phospholipase D to their alkyl phosphates and choline, and the alkyl phosphates were subsequently metabolised by phosphatase to yield the alcohols and inorganic phosphate. These studies failed to detect any metabolism of either SRI or HPC by phospholipase C, and the metabolism of platelet-activating factor (PAF) by this enzyme was not inhibited by the addition of either compound. The cytotoxicity of SRI, the related compound HPC and their metabolites was determined in vitro using three cell lines. Cytotoxicity was measured by analysis of cell growth kinetics, MTT assay and lactate dehydrogenase release. Closely similar results were obtained in the JB1 rat hepatoma cell line, in the non-transformed BL8 rat hepatocyte cell line, and in A549 human lung adenocarcinoma cells. SRI was the most toxic of the compounds analysed, the concentration required to produce 50% toxicity or growth inhibition (IC50) being 6–9 μm. The putative metabolite of SRI, 2,2′-bis(hydroxymethyl)tetrahydrofuran, and the known metabolites [2′-(octadecyloxymethyl)tetrahydrofuran-2′-yl]methyl phosphate and 2-hydroxymethyl-2-octadecyloxymethyltetrahydrofuran exhibited IC50 values of >200, >100 and 40–70 μm, respectively, consistent with metabolic detoxification. HPC was more cytotoxic (IC50, 37 μm) than its phosphate metabolite (IC50, 140 μm), but its toxicity was similar to that of its metabolite hexadecanol (IC50, 28 μm), suggesting that only the former metabolic route leads to detoxification.
Similar content being viewed by others
Abbreviations
- BnP:
-
benzylphosphonate
- ET18O CH3 :
-
2-methyl-1-octadecyl-glycero-3-phosphocholine
- HPC:
-
hexadecylphosphocholine
- LDH:
-
lactate dehydrogenase
- MES:
-
morpholinoethanesulphonate
- MTT:
-
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-2H-tetrazolium bromide
- NMR:
-
nuclear magnetic resonance
- PAF:
-
platelet-activating factor, 2-acetyl-l-octadecyl-glycero-3-phosphocholine
- PBS:
-
phosphate-buffered saline
- SRI 62-834:
-
SRI, [2′-(octadecyloxymethyl)tetrahydrofuran-2′-yl]methyl 2-[N,N,N-trimethylammonio]ethylphosphate
References
Amouroux R, Chastrette F, Chastrette M (1981) Synthèse de polyéthers polycliques: 1. Stéréosélectivité de l'heterocyclisation d'alcools tétrahydrofurfuryliques γ, δéthyléniques par les sels mercuriques. J Heterocyc Chem 18: 565
Bazill GW, Dexter TM (1989) An antagonist to platelet activating factor counteracts the tumouricidal action of alkyl lysophospholipids. Biochem Pharmacol 38: 374
Bazill GW, Dexter TM (1990) Role of endocytosis in the action of ether lipids in WEH1-3B, HL60 and FDCP-mix A4 cells. Cancer Res 50: 7505
Berdel WE, Bausert WR, Weltzein HU, Modolell ML, Widman KH, Munder PG (1980) The influence of alkyl-lysophospholipids and lysophospholipid-activated macrophages on the development of metastasis of 3-Lewis lung carcinoma. Eur J Cancer 16: 1199
Berdel WE, Bausert WRE, Fink U, Rastetter J, Munder PG (1981) Anti-tumour action of alkyl-lysophospholipids. Anticancer Res 1: 345
Bergmeyer HU, Bernt E (1974) Lactate dehydrogenase. In: Bergmeyer HU (ed) Methods of enzymatic analysis, vol II. Academic Press, New York, p 574
Bertz RT (1963) Furtural diacetate (2-furanmethanediol, diacetate). Org Synth Coll IV: 489
Bomalaski JS, Clark MA (1987) The effect ofsn-2 fatty acid substitution on phospholipase C enzyme activities. Biochem J 244: 497
Breiser A, Kim D-J, Fleer EA, Damenz W, Drube A, Berger M, Nagel GA, Eibl H, Unger C (1987) Distribution and metabolism of hexadecylphosphocholine in mice. Lipids 22: 95
Dive C, Watson JV, Workman P (1991) Multiparametric flow cytometry of the modulation of tumor cell membrane permeability by developmental antitumor ether lipid SRI 62-834 in EMT6 mouse mammary tumor and HL-60 human promyelocytic leukemia cells. Cancer Res 51: 799
Eftax DSP, Dunlop AP (1961) New diols in the furan and pyran series. J Org Chem 26: 2106
El-Sayed MY, DeBose CD, Coury LA, Roberts MF (1985) Sensitivity of phospholipase C (Bacillus cereus) activity to phosphatidylcholine structural modifications. Biochim Biophys Acta 837: 325
Fleer EAM, Unger C, Kim D-J, Eibl H (1987) Metabolism of ether phospholipids and analogs in neoplastic cells. Lipids 22: 856
Houlihan WJ, Lee ML, Munder PG, Nemecek GM, Handley DA, Winslow CM, Happy J, Jaeggi C (1987) Antitumour activity of SR 62-834, a cyclic ether analog of ET-18-OCH3. Lipids 22: 884
Lazenby CM, Thompson MG, Hickman JA (1990) Elevation of leukemic cell intracellular calcium by ether lipid SRI62-834. Cancer Res 50: 3327
Manson MM, Legg RF, Watson JV, Green JA, Neal GE (1981) An examination of the relative resistances to aflatoxin B1 and susceptibilities to γ-glutamyl p-phenylene diamine mustard of γ-glutamyl transferase negative and positive cell lines. Carcinogenesis 2: 661
Mosmann T (1983) Rapid colorimetric assay for cellular growth and survival: applications to proliferation and cytotoxicity assays. J Immunol Methods 65: 55
Noseda A, White JG, Godwin PL, Jerome WG, Modest EJ (1989) Membrane damage in leukemic cells induced by ether and ester lipids: an electron microscopic study. Exp Mol Pathol 50: 69
Okamoto S, Olson AC, Vogler WR, Winton EF (1987) Purging leukemic cells from simulated human remission marrow with alkyllysophospholipids. Blood 69: 1381
Okayasu T, Hoshii K, Seyama K, Ishibashi T, Imai Y (1986) Metabolism of platelet-activating factor in primary cultured adult rat hepatocytes by a new pathway involving phospholipase C and alkyl monooxygenase. Biochim Biophys Acta 876: 58
Perich JW, Johns RB (1988) Di-tert-butylN,N-diethylphosphoramidite. A new phosphitylating agent for the efficient phosphorylation of alcohols. Synthesis 142
Seewald MJ, Olsen RA, Sehgal I, Melder DC, Modest EJ, Powis G (1990) Inhibition of growth factor-dependent inositol phosphate Ca2+ signaling by antitumor ether lipid analogues. Cancer Res 50: 4458
Still WC, Kahn M, Mitra A (1978) Rapid chromatographic technique for preparative separations with moderate resolution. J Org Chem 43: 2923
Twentyman PR, Luscombe M (1987) A study of some variables in a tetrazolium dye (MTT) based assay for cell growth and chemosensitivity. Br J Cancer 56: 279
Überall F, Oberhuber H, Maly K, Zaknun J, Demuth L, Grunicke HH (1991) Hexadecylphosphocholine inhibits inositol phosphate formation and protein kinase C activity. Cancer Res 51: 807
Unger C, Eibl H, Kim DJ, Fleer EA, Koetting J, Bartsch HH, Nagel GA, Pfitzenmaier K (1987) Sensitivity of leukemia cell lines to cytotoxic alkyl-lysophospholipids in relation toO-alkyl cleavage enzyme activities. J Natl Cancer Inst 78: 219
Unger C, Damenz W, Fleer EA, Kim DJ, Breiser A, Hilgard P, Engel J, Nagel G, Eibl H (1989) Hexadecylphosphocholine, a new ether lipid analogue. Studies on the antineoplastic activity in vitro and in vivo. Acta Oncol 28: 213
Vallari DS, Smith ZL, Snyder F (1988) HL-60 cells become resistant towards antitumor ether-linked phospholipids following differentiation into a granulocytic form. Biochem Biophys Res Commun 156: 1
Wilcox RW, Wykle RL, Schmitt JD, Daniel LW (1987) The degradation of platelet-activating factor and related lipids: susceptibility to phospholipases C and D. Lipids 22: 800
Workman P (1991) Antitumour ether lipids: endocytosis as a determinant of cellular sensitivity. Cancer Cells 3: 315
Zheng B, Oishi K, Shoji M, Eibl H, Berdel WE, Hajdu J, Vogler WR, Kuo JF (1990) Inhibition of protein kinase C, (sodium plus potassium)-activated adenosine triphosphatase, and sodium pump by synthetic phospholipid analogues. Cancer Res 50: 3025
Author information
Authors and Affiliations
Additional information
This work was supported by CRC programme grant SP1518 and by an MRC postgraduate fellowship (awarded to F. E. B.). One of the authors (S. E.) is a Lister Institute Fellow and another (C. D.) was supported by the Wolfson Trust
Rights and permissions
About this article
Cite this article
Bishop, F.E., Dive, C., Freeman, S. et al. Is metabolism an important arbiter of anticancer activity of ether lipids? metabolism of SRI 62-834 and hexadecylphosphocholine by [31P]-NMR spectroscopy and comparison of their cytotoxicities with those of their metabolites. Cancer Chemother. Pharmacol. 31, 85–92 (1992). https://doi.org/10.1007/BF00685092
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00685092