Abstract
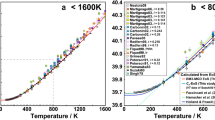
The equilibrium between spinel lherzolite and garnet lherzolite has been experimentally determined in the CaO-MgO-Al2O3-SiO2 system between 800° and 1,100° C. In confirmation of earlier work and predictions from thermodynamic data, it was found that theP-T slope of the reaction was close to zero, the equilibrium ranging from 16.1 kb at 800° C to 18.7 kb at 1,100° C (±0.3 kb).
The addition of Cr2O3 to the system raised the stability field of spinel to higher pressures. It was found that the pressure at which both garnet and spinel could exist with olivine+orthopyroxene+clinopyroxene in the system CMAS −Cr2O3 could best be described by the empirical relationship:
whereP 0 is the equilibrium pressure for the univariant reaction in the Cr2O3-free system,α is a constant apparently independent of temperature with a value of 27.9 kilobars, andX spCr is the mole fraction of chromium in spinel.
Use was made of the extensive literature on Mg-Fe2+ solid solutions to quantitatively derive the effect of Fe2+ on the equilibrium. The effect of other components (Fe3+, Na) was also considered.
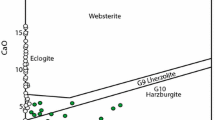
The equilibrium can be used as a sensitive geobarometer for rocks containing the five phases ol+opx+cpx+gt+sp, and thus provides the only independent check presently available for the more widely applicable geobarometer which uses the alumina content of orthopyroxene in equilibrium with garnet.
Similar content being viewed by others
References
Boyd FR (1973) A pyroxene geotherm. Geochim. Cosmochim Acta 37:2533–2546
Boyd FR, England JL (1960) Apparatus for phase equilibrium measurements at pressures up to 50kb and temperatures up to 1,750° C. J. Geophys Res 65:741–748
Boyd FR, Finger LW (1975) Homogeneity of minerals in mantle rocks from Lesotho. Carnegie Inst Washington Yearb 74:519–525
Cooley RF, Reed JS (1972) Equilibrium cation distribution in NiAl2O4, CuAl2O4 and ZnAl2O4 spinels. J Am Ceram Soc 55:395–398
Cressey G, Schmid R, Wood BJ (1978) Thermodynamic properties of almandine-grossular garnet solid solutions. Contrib Mineral Petrol 67:397–404
Engi M (1978) Mg-Fe exchange equilibria among Al-Cr spinel, olivine, orthopyroxene and cordierite. PhD Thesis, Swiss Federal Institute of Technology, Zurich
Ferguson J, Sheraton JW (1979) Petrogenesis of Kimberlitic rocks and associated xenoliths of southeastern Australia. In: FR Boyd and HOA Meyer (eds) Proceedings of the 2nd Int Kimberlite Conf, Vol 1. Am Geophys Union
Fisher GW, Medaris LG (1969) Cell dimensions and X-ray determinative curve for synthetic Mg-Fe olivines. Am Mineral 54:741–753
Fyfe WS (1960) Hydrothermal synthesis and determination of equilibrium between minerals in the subliquidus region. J Geol 68:553–566
Hafner S, Laves F (1961) Ordnung/Unordnung und Ultrarotabsorption. III. Die Systeme MgAl2O4-Al2O3 und MgAl2O4-LiAl5O8. Z Kristallogr Mineral 115:321–330
Hamilton DL, Henderson CMB (1968) The preparation of silicate compositions by a gelling method. Mineral Mag 36:832–838
Herzberg CT (1978) Pyroxene geothermometry and geobarometry: experimental and thermodynamic evaluation of some subsolidus phase relations involving pyroxenes in the system CaO-MgO-Al2O3-SiO2. Geochim Cosmochim Acta 42:945–957
Irvine TN (1965) Chromian spinel as a petrogenetic indicator. I. Theory. Can J Earth Sci 2:648–672
Irvine TN (1967) Chromian spinel as a petrogenetic indicator. II. Petrologic applications. Can J Earth Sci 4:71–103
Jenkins DM, Newton RC (1979) Experimental determination of the spinel peridotite to garnet peridotite inversion at 900° C and 1,000° C in the system CaO-MgO-Al2O3-SiO2, and at 900° C with natural garnet and olivine. Contrib Mineral Petrol 68:407–419
Kushiro I, Syono Y, Akimoto S (1967) Effect of pressure on garnetpyroxene equilibrium in the system MgSiO3-CaSiO3-Al2O3. Earth Planet Sci Lett 2:460–464
Kushiro I, Yoder HS (1966) Anorthite-forsterite and anorthite-enstatite reactions and their bearing on the basalt-eclogite transformation. J Petrol 7:337–362
Larimer JW (1968) Experimental studies on the system Fe-MgO-SiO2-O2, and their bearing on the petrology of chondritic meteorites. Geochim Cosmochim Acta 32:1187–1207
MacGregor ID (1965) Stability fields of spinel and garnet peridotites in the synthetic system MgO-CaO-A12O3-SiO2. Carnegie Inst Washington Yearb 64:126–134
MacGregor ID (1970) The effect of CaO, Cr2O3, Fe2O3 and Al2O3 on the stability of spinel and garnet peridotites. Phys Earth Planet Interiors 3:372–377
Matsui Y, Nishizawa O (1974) Iron II-magnesium exchange equilibrium between olivine and calcium-free pyroxene over a temperature range 800° C to 1,300° C. Bull Soc Fr Mineral Cristallogr 97:122–130
Matsui Y, Syono Y, Akimoto S, Kitayama K (1968) Unit cell dimensions of some synthetic orthopyroxene group solid solutions. Geochem J 2:61–70
Medaris LG Jr (1969) Partitioning of Fe++ and Mg++ between coexisting synthetic olivine and orthopyroxene. Am J Sci 267:945–968
Mori T, Green DH (1978) Laboratory duplication of phase equilibria observed in natural garnet lherzolites. J. Geol 86:83–97
Navrotsky A, Kleppa OJ (1967) The thermodynamics of cation distribution in simple spinels. J Inorg Nucl Chem 29:2701–2714
Nehru CE (1976) Pressure dependence of the enstatite limb of the enstatite-diopside solvus. Am Mineral 61:578–581
Newton RC, Charlu RV, Kleppa OJ (1977) Thermochemistry of high pressure garnets and clinopyroxenes in the system CaO-MgO-Al2O3-SiO2. Geochim Cosmochim Acta 41:369–377
Nixon PH, Boyd FR (1973) Petrogenesis of the granular and sheared ultrabasic nodule suite in kimberlites. In: PH Nixon (ed), Lesotho Kimberlites. Lesotho National Development Corporation
Nixon PH, Boyd FR (1979) Garnet bearing lherzolites and discrete nodule suites from the Malaita alnoite, Solomon Islands, SW Pacific, and their bearing on oceanic mantle composition and geotherm. In: FR Boyd, HOA Meyer (eds) Proceedings of the Second International Kimberlite Conference, Vol II. Am Geophys Union
Obata M (1976) The solubility of Al2 O in orthopyroxene in spinel and plagioclase peridodites and spinel pyroxenite. Am Mineral 61:804–816
O'Hara MJ, Richardson SW, Wilson G (1971) Garnet-peridotite stability in crust and mantle. Contrib Mineral Petrol 32:48–68
O'Neill HStC, Wood BJ (1979) An experimental study of Fe-Mg partitioning between garnet and olivine and its calibration as a geothermometer. Contrib Mineral Petrol 70:59–70
Ringwood AE (1962) A model for the upper mantle. J Geophys Res 67:857–867
Robie RA, Hemingway B, Fisher JR (1978) Thermodynamic properties of minerals and related substances at 298-15 K and 1 bar (105 pascals) pressure and at higher temperatures. US Geol Surv Bull 1452
Roeder PL, Campbell IH, Jamieson HE (1979) A reevaluation of the olivine-spinel geothermometer. Contrib Mineral Petrol 68:325–334
Schmalzried H (1961) Röntgenographische Untersuchung der Kationenverteilung in Spinellphasen. Z Phys Chem NF 28:203–219
Schmid R, Cressey G, Wood BJ (1978) Experimental determination of univariant equilibria using divariant solid-solution assemblages. Am Mineral 63:511–515
Schmocker U, Boesch HR, Waldner F (1972) A direct determination of cation disorder in MgAl2O4 spinel by ESR. Phys Lett 40A:237–238
Thompson JB Jr (1967) Thermodynamic properties of simple solutions. In: PH Abelson (ed), Researches in geochemistry, Vol 2. New York, Wiley
Turnock AC, Lindsley DH, Grover JE (1973) Synthesis and unit cell parameters of Ca-Mg-Fe pyroxenes. Am Mineral 58:50–59
Warshaw I, Keith ML (1954) Solids solution and chromium oxide loss in part of the system MgO-Al2O3-Cr2O3-SiO2. J Am Ceram Soc 37:161–168
Wells PRA (1977) Pyroxene thermometry in simple and complex systems. Contrib Mineral Petrol 62:129–139
Wood BJ (1974) The solubility of alumina in orthopyroxene coexisting with garnet. Contrib Mineral Petrol 46:1–15
Wood BJ (1978) The influence of Cr2O3 on the relationships between spinel- and garnet-peridotites. Extended Abstr 2nd Int Kimberlite Conf
Wood BJ, Nicholls J (1978) The thermodynamic properties of reciprocal solid solutions. Contrib Mineral Petrol 66:389–400
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
O'Neill, H.S.C. The transition between spinel lherzolite and garnet lherzolite, and its use as a Geobarometer. Contr. Mineral. and Petrol. 77, 185–194 (1981). https://doi.org/10.1007/BF00636522
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00636522