Abstract
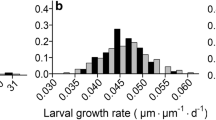
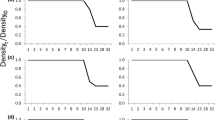
Two subspecies of the predatory aquatic salamanderNotophthalmus, N. viridescens viridescens andN. v. dorsalis, differ in adult body size and geographic distribution. We tested whether experimental populations of the two predator subspecies differed in their effects on prey populations ofB. americanus, and whether observed differences in predator body size were genetic and/or environmentally induced. We compared the effects of predation by bothNotophthalmus subspecies on larvalBufo americanus by experimentally manipulating the densities (0, 2, or 4 newts/m3) and subspecies ofNotophthalmus (N. v. viridescens orN. v. dorsalis) added to artificial ponds. BothNotophthalmus subspecies significantly reducedB. americanus survival, but differed significantly in this effect. FewerBufo survived with the larger subspecies,N. v. viridescens, than with the smallerNotophthalmus subspecies,N. v. dorsalis. TheNotophthalmus subspecies differed in their patterns of adult and larval growth. Adults of the smaller subspecies,N. v. dorsalis, had a significantly higher growth rate than the larger subspecies,N. v. viridescens, under common environmental conditions, suggesting that differences in predator size were partly genetic, rather than entirely environmentally induced. LarvalN. v. dorsalis metamorphosed significantly later in the season than larvae ofN. v. viridescens, suggesting that larvalN. v. dorsalis had a lower growth rate than larvalN. v. viridescens. Differences in adult and larval growth, together with differences in the minimum adult size observed in natural populations, suggest that differences in the rate or duration of pre-adult growth may contribute substantially to observed differences in size.
Similar content being viewed by others
References
Alford RA (1989) Variation in predator phenology affects predator performance and prey community composition. Ecology 70:206–219
Andrews RM (1982) Patterns of growth in reptiles. In: Gans C, Pough FH (eds) Biology of the reptiles, vol 13: Physiological ecology. Academic Press, London, pp 273–320
Arnold SJ (1981) Behavioral variation in natural populations. II. The inheritance of a feeding response in crosses between geographic races of the garter snake,Thamnophis elegans. Evolution 35:510–515
Berven KA, Gill DE (1983) Interpreting geographic variation in life-history traits. Amer Zool 23:85–97
Briand F (1983) Environmental control of food web structure. Ecology 64:253–263
Bristow CE (1991) Interactions between phylogenetically distant predators:Notophthalmus viridescens andEnneacanthus obesus. Copeia 1991:1–8
Brockelman WY (1969) An analysis of density effects and predation inBufo americanus tadpoles. Ecology 50:632–644
Brophy TE (1980) Food habits of sympatric larvalAmbystoma tigrinum andNotophthalmus viridescens. J Herpetol 14:1–6
Bruce RC (1990) An explanation for differences in body size between two desmognathine salamanders. Copeia 1990:1–9
Clausen J, Keck DD, Heisey WM (1948) Experimental studies on the nature of species. III. Environmental responses of climatic races ofAchillea. Carnegie Inst Wash Publ 581:1–129
Conant R, Collins JT (1991) A field guide to the reptiles and amphibians of eastern and central North America, 3rd edn. Houghton Miflin, Boston
Fauth JE, Resetarits WJ Jr (1991) Interactions between the salamanderSiren intermedia and the keystone predatorNotophthalmus viridescens. Ecology 72:827–838
Forester DC, Lykens DV (1991) Age structure in a population of red-spotted newts from the Allegheny Plateau of Maryland. J Herpetol 25:373–376
Freund RJ, Littell RC (1981) SAS for linear models: a guide to the ANOVA and GLM procedures. SAS Institute Inc., Cary, NC
Gerwien RW, John-Alder HB (1992) Growth and behavior of thyroid-deficient lizards (Sceloporus undulatus). Gen Comp Endocrinol 87:312–324
Gill DE (1978) The metapopulation ecology of the red-spotted newt,Notophthalmus viridescens (Rafinesque). Ecol Monogr 48:145–166
Gill DE (1979) Density dependence and homing behavior in adult red-spotted newtsNotophthalmus viridescens (Rafinesque). Ecology 60:800–813
Hairston NG (1980) Evolution under interspecific competition: field experiments on terrestrial salamanders. Evolution 34:409–420
Harris RN (1987a) Density-dependent paedomorphosis in the salamanderNotophthalmus viridescens dorsalis. Ecology 68:705–712
Harris RN (1987b) An experimental study of population regulation in the salamander,Notophthalmus viridescens dorsalis (Urodela: Salamandridae). Oecologia 71:280–285
Harris RN, Alford RA, Wilbur HM (1988) Density and phenology of adult and larvalNotophthalmus viridescens dorsalis in a natural pond. Herpetologica 44:234–242
Healy WR (1973) Life history variation and the growth of juvenileNotophthalmus viridescens from Massachusetts. Copeia 1973:641–647
Healy WR (1974) Population consequences of alternative life histories inNotophthalmus v. viridescens. Copeia 1974:221–229
MacArthur RH (1972) Geographical ecology. Harper and Row, New York
Martof BS, Palmer WM, Bailey RJ, Harrison JR III (1980) Amphibians and reptiles of the Carolinas and Virginia. The University of North Carolina Press, Chapel Hill, NC
May RM (1974) Stability and complexity in model ecosystems. Princeton University Press, Princeton, NJ
Morin PJ (1981) Predatory salamanders reverse the outcome of competition among three species of anuran tadpoles. Science 212:1284–1286
Morin PJ (1983a) Predation, competition, and the composition of larval anuran guilds. Ecol Monogr 53:119–138
Morin PJ (1983b) Competitive and predatory interactions in natural and experimental populations ofNotophthalmus viridescens dorsalis andAmbystoma tigrinum. Copeia 1983:628–639
Morin PJ (1986) Interactions between intraspecific competition and predation in an amphibian predator-prey system. Ecology 67:713–720
Morin PJ (1987) Predation, breeding asynchrony, and the outcome of competition among treefrog tadpoles. Ecology 68:675–683
Morin PJ, Wilbur HM, Harris RN (1983) Salamander predation and the structure of experimental communities: responses ofNotophthalmus and microcrustacea. Ecology 64:1430–1436
Paine RT (1976) Size-limited predation: an observational and experimental approach with theMytilus-Pisaster interaction. Ecology 57:858–873
Paine RT (1980) Food webs: linkage, interaction strength and community infrastructure. J Anim Ecol 49:667–685
Persson L (1988) Asymmetries in competitive and predatory interactions in fish populations. In: Ebenman B, Persson L (eds) Size-structured populations. Springer, Berlin Heidelberg New York, pp 203–218
Pianka ER (1966) Latitudinal gradients in species diversity: a review of the concepts. Am Nat 100:33–46
Polis GA (1988) Exploitation competition and the evolution of interference, cannibalism, and intraguild predation in age/sizestructured populations. In: Ebenman B, Persson L (eds) Sizestructured populations. Springer, Berlin Heidelberg New York, pp 185–202
Reilly SM (1990) Biochemical systematics and evolution of the eastern North American newts, genusNotophthalmus (Caudata: Salamandridae). Herpetologica 46:51–59
Reznick DA, Bryga H, Endler JA (1990) Experimentally induced life-history evolution in a natural population. Nature 346:357–35
Rice WR (1990) A consensus combined p-value test and the family-wide significance of component tests. Biometrics 46:303–308
Roughgarden JR, Gaines S, Possingham H (1988) Recruitment dynamics and complex life cycles. Science 241:1460–1466
Scheiner SM (1993) MANOVA: multiple response variables and multiple species interactions. In: Scheiner SM, Gurevitch J, (eds) Design and analysis of ecological experiments. Chapman and Hall, New York, pp 95–112
Semlitsch RD, Gibbons JW (1988) Fish predation in size-structured populations of treefrog tadpoles. Oecologia 75:321–326
Semlitsch RD, Gibbons JW (1990) Effects of egg size on success of larval salamanders in complex aquatic environments. Ecology 71:1789–1795
Smith DC (1983) Factors controlling tadpole populations of the chorus frog (Pseudacris triseriata) on Isle Royale, Michigan. Ecology 64:501–510
Sokal RR, Rohlf FJ (1981) Biometry, 2nd edn. W.H. Freeman and company, New York
Tabachnik WJ (1977) Geographic variation of five biochemical polymorphisms inNotophthalmus viridescens. J Hered 68:117–122
Travis J, Keen WH, Juilianna J (1985) The role of relative body size in a predator-prey relationship between dragonfly naiads and larval anurans. Oikos 45:59–65
Underwood AJ, Fairweather PG (1989) Supply-side ecology and benthic marine assemblages. Trends Ecol Evol 4:16–20
Werner EE (1986) Amphibian metamorphosis: growth rate, predation risk, and the optimal size at transformation. Am Nat 128:319–341
Wilbur HM (1987) Regulation of structure in complex systems: experimental temporary pond communities. Ecology 68:1437–1452
Wilbur HM (1988) Interactions in size-structured populations: from individual behavior to ecosystem dynamics. In: Ebenman B, Persson L (eds) Size-structured populations. Springer, Berlin Heidelberg, New York, pp 157–183
Wilbur HM, Fauth JE (1990) Experimental aquatic food webs: interactions between two predators and two prey. Am Nat 135:176–204
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Kurzava, L.M., Morin, P.J. Consequences and causes of geographic variation in the body size of a keystone predator,Notophthalmus viridescens . Oecologia 99, 271–280 (1994). https://doi.org/10.1007/BF00627739
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00627739