Summary
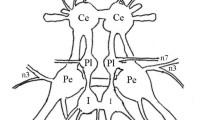
The metacerebral giant (MCG) neurons of the molluskPleurobranchaea have been analyzed using a wide range of methods (cobalt staining, histochemical, biophysical and electrophysiological) on several types of preparations (isolated nervous systems, semi-intact preparations, and behaving whole-animal preparations). The MCG is serotonergic. The bilaterally-symmetrical neurons have somata in the anterior brain. Each MCG neuron sends an axon out the ipsilateral mouth nerve of the brain and also into the ipsilateral cerebrobuccal connective which descends to the buccal ganglion. The descending axon sends one or more branches out most buccal nerves.
The MCG makes mono- and polysynaptic chemical excitatory and inhibitory connections with identified feeding motoneurons in the buccal ganglion. In quiescent preparations (isolated CNS or semi-intact), MCG stimulation caused coordinated eversion activity followed immediately by withdrawal activity. During an ongoing feeding rhythm (spontaneous output or induced by stimulation of the stomatogastric nerve), tonic stimulation of one or both MCG's at physiological discharge frequencies typically caused a significant increase in the frequency of the rhythm, and usually emphasized the eversion component at the expense of the withdrawal component. Phasic stimulation of one or both MCG's at physiological discharge frequencies and in normal discharge patterns (bursts; see below) accelerated and phaselocked the feeding rhythm.
The MCG neurons receive synaptic feedback from identified neurons in the feeding network. Brain motoneurons are reciprocally coupled with the MCG by non-rectifying electrical synapses, while buccal ganglion neurons (the previously identified corollary discharge neurons) inhibit the MCG. Recordings from the MCG during cyclic feeding show that it discharges cyclically and that its membrane potential oscillates in phase with the feeding rhythm, presumably reflecting the above synaptic feedback. Two biophysical properties of the MCG membrane, namely anomalous rectification and postspike conductance increase, are presumed to contribute to the MCG's oscillatory activity.
Chemosensory (food stimuli) and mechanosensory inputs from the oral veil excite the MCG's. In whole-animal preparations, these sensory inputs typically cause discharge in the MCG's and other descending neurons, accompanied by feeding motor output.
The data collectively suggest that the MCG's ofPleurobranchaea are members of a population of neurons that normally function to command (i.e., arouse, initiate and sustain) the rhythmic feeding behavior. The demonstrated central feedback to the MCG is presumed to amplify these command functions.
Similar content being viewed by others
References
Berry, M.S., Cottrell, G.A., Pentreath, V.W.: Synaptic connexions of serotonin-containing neurones inPlanorbis corneus. J. Physiol. (Lond.)241, 2–3P (1975)
Connor, J.A., Stevens, C.F.: Voltage clamp studies of a transient outward membrane current in gastropod neural somata. J. Physiol. (Lond.)213, 21–30 (1971)
Cottrell, G.A., Macon, J.B.: Synaptic connexions of two symmetrically placed giant serotonin-containing neurones. J. Physiol. (Lond.)236, 435–464 (1974)
Cottrell, G.A., Macon, I., Szczepaniak, A.C.: Glutamic acid mimicking of synaptic inhibition on the giant serotonin neurone of the snail. Brit. J. Pharmacol.45, 684–688 (1972)
Cottrell, G.A., Osborne, N.N.: Subcellular localization of serotonin in an identified serotonin-containing neuron. Nature (Lond.)225, 470–472 (1970)
Davis, W.J.: Organizational concepts in the central motor networks of invertebrates. In: Neural control of locomotion (eds. R.M. Herman, S. Grillner, P.S.G. Stein, D.G. Stuart), pp. 265–292. New York: Plenum 1976
Davis, W.J.: The command neuron. In: Identified neurons and behavior of arthropods (ed. G. Hoyle). New York: Plenum (in press) 1977
Davis, W.J., Kennedy, D.: Command neurons controlling swimmeret movements in the lobster. I. Types of effects on motoneurons. J. Neurophysiol.35, 1–12 (1972a)
Davis, W.J., Kennedy, D.: Command neurons controlling swimmeret movements in the lobster. II. Interaction of effects on motoneurons. J. Neurophysiol.35, 13–19 (1972b)
Davis, W.J., Mpitsos, G.: Behavioral choice and habituation in the marine molluskPleurobranchaea californica MacFarland (Gastropoda, Opisthobranchia). Z. vergl. Physiol.75, 207–232 (1971)
Davis, W.J., Mpitsos, G.J., Pinneo, J.M.: The behavioral hierarchy of the molluskPleurobranchaea. I. The dominant position of the feeding behavior. J. comp. Physiol.90, 207–224 (1974a)
Davis, W.J., Mpitsos, G.J., Pinneo, J.M.: The behavioral hierarchy of the molluskPleurobranchaea. II. Hormonal suppression of feeding associated with egg laying. J. comp. Physiol.90, 225–243 (1974b)
Davis, W.J., Mpitsos, G.J., Siegler, M.V.S., Pinneo, J.M., Davis, K.B.: Neuronal substrates of behavioral hierarchies and associative learning inPleurobranchaea. Amer. Zool.13, 1037–1049 (1974)
Davis, W.J., Siegler, M.V.S., Mpitsos, G.J.: Distributed neuronal oscillators and efference copy in the feeding system ofPleurobranchaea. J. Neurophysiol.36, 258–274 (1973)
Hanley, M.R., Cottrell, G.A., Emson, P.C., Fonnum, F.: Enzymatic synthesis of acetylcholine by a serotonin-containing neurone fromHelix. Nature (Lond.)251, 631–633 (1974)
Junge, D., Stevens, C.L.: Cyclic variation of potassium conductance in a burst-generating neurone inAplysia. J. Physiol. (Lond.)235, 155–181 (1973)
Kandel, E.R., Tauc, L.: Input organization of two symmetrical giant cells in the snail brain, J. Physiol. (Lond.)183, 269–286 (1966a)
Kandel, E.R., Tauc, L.: Anomalous rectification and its consequences for synaptic transmission. J. Physiol. (Lond.)183, 287–304 (1966b)
Lindvall, O., Björklund, A.: The glyoxylic acid fluorescence histochemical method: a detailed account of the methodology for the visualization of central catecholamine neurons. Histochem.39, 97–127 (1974)
Mpitsos, G.J., Collins, S.: Learning: Rapid aversive conditioning in the gastropod mollusk,Pleurobranchaea. Science188, 954–957 (1975)
Mpitsos, G.J., Davis, W.J.: Learning: Classical and avoidance conditioning in the molluscPleurobranchaea. Science180, 317–320 (1973)
Paupardin-Tritsch, D., Gerschenfeld, H.M.: Transmitter role of serotonin in identified synapses inAplysia nervous system. Brain Res.58, 529–536 (1973)
Pentreath, V.W.: Effects of stimulating a central giant serotonin-containing neuron on peripheral muscles in the snailHelix pomatia. Experientia (Basel)29, 540–542 (1975)
Pentreath, V.W., Cottrell, G.A.: Anatomy of an identified serotonin neurone studied by means of injection of tritiated ‘transmitter”. Nature (Lond.)250, 655–658 (1974)
Pitman, R.N., Tweedle, C.W., Cohen, M.J.: Branching of central neurons: intracellular cobalt injection for light and electron microscopy. Science176, 384–385 (1972)
Siegler, M.V.S., Mpitsos, G.J., Davis, W.J.: Motor organization and generation of rhythmic feeding output in buccal ganglion ofPleurobranchaea. J. Neurophysiol.37, 1173–1196 (1974)
Weinreich, D., McCaman, M.W., McCaman, R.E., Vaughn, J.E.: Chemical enzymatic and ultrastructural characterization of 5-hydroxytryptamine-containing neurons from the ganglia ofAplysia californica andTritonia diomedia. J. Neurochem.30, 969–976 (1973)
Weiss, K.R., Cohen, J., Kupfermann, I.: Potentiation of muscle contraction: A possible modulatory function of an identified serotonergic cell inAplysia. Brain Res.99, 381–386 (1975)
Weiss, K.R., Kupfermann, I.: Homology of giant cerebral cells inAplysia to the metacerebral cells of pulmonate molluscs. Society for Neuroscience Abstracts, 4th annual meeting, 1974
Willows, A.O.D., Dorsett, D.A., Hoyle, G.: Neuronal basis of behavior inTritonia. J. Neurobiol.4, 207–237 (1973)
Willows, A.O.D., Hoyle, G.: Neuronal network triggering a fixed action pattern. Science166, 1549–1551 (1969)
Author information
Authors and Affiliations
Additional information
Supported by an NIH Postdoctoral Fellowship (1 F22 NS00511) to R.G. and NIH Research Grants NS 09050 and MH 23254 to W.J.D. We thank Kathryn H. Britton for histological assistance. We also thank Mark P. Kovac, who produced the records of Figures 8 and 18, for permission to reproduce them here.
Rights and permissions
About this article
Cite this article
Gillette, R., Davis, W.J. The role of the metacerebral giant neuron in the feeding behavior ofPleurobranchaea . J. Comp. Physiol. 116, 129–159 (1977). https://doi.org/10.1007/BF00605400
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF00605400