Summary
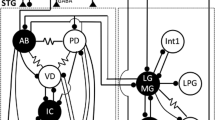
The ionic requirements for bursting activity have been investigated in the electrically coupled PD-AB cells group of the Stomatogastric ganglion in the lobster.
The passive electrical properties and coupling parameters have been determined in either current or voltage clamp conditions. In voltage clamped cells, the current displayed slow inward transients with superimposed fast transients corresponding respectively to the slow waves and spikes of the coupled undamped cells. The amplitude and frequency of the slow transients were reduced upon hyperpolarization.
Cyclic conductance changes were observed with short current pulses, the coupling ratio also changes cyclically being lower during the bursts and slowly increasing during the interburst interval.
The slow wave amplitude increased in low K-saline. The post-burst hyperpolarization but not the top level of the wave behaved like a potassium electrode for [K]o higher than 10 mM/l.
TEA at low concentration (1 to 5 mM/l) increased the slow wave amplitude by lifting its top level by 10 to 20 mV. The post-burst hyperpolarization remained almost unchanged and its K-dependence was not altered by TEA.
Low Na-saline reduced the slow wave amplitude (6 to 11 mV per decade). The Na-dependence increased in the presence of TEA. Slow waves devoid of spikes persisted in 10% Na saline containing TEA. 10−9 M/1 TTX blocked the spikes. 10−7 M/1 TTX blocked the slow waves.
Mg-free saline had no effect on the slow wave. In Ca-free saline the cells depolarized and the bursting activity tended to vanish. Repolarization with current led to long lasting slow waves deprived of post-burst hyperpolarization. The bursting ceased when EDTA was added to the Ca-free saline.
Cobalt (up to 10 mM/l) was similar to Ca-free saline in its effects; lengthening of the wave and blockage of the repolarization. Replacing Ba for Ca produced large (up to 70 mV) slow waves which were reduced by Co and Ca.
Bistable states were observed in various experimental conditions. It is concluded that the slow waves are produced by activation of sodium and calcium currents. The amplitude of the slow wave is modulated by the simultaneous activation of a TEA-sensitive K current. The repolarization is caused by increased K current activated by the inward calcium current. The slow pacemaker potential in the interburst interval corresponds to the slow disappearance of the K current.
Similar content being viewed by others
References
Arvanitaki A, Chalazonitis N (1967) Electrical properties and temporal organization in oscillatory neurons. In: Salanki J (ed) Neurobiology of invertebrates. Plenum Press, New-York, pp 169–199
Ayrapetyan SN (1973) On the regulation of the mechanism of rhythmic activity ofHelix neurones. In: Salanki J (ed) Neurobiology of invertebrates. Akademiai Kiado, Budapest, pp 81–92
Barker JL, Gainer H (1975) Studies on bursting pacemaker potential activity in molluscan neurons. I. Membrane properties and ionic contributions. Brain Res 84:461–477
Barker JL, Smith TG (1978) Electrophysiological studies of molluscan neurons generating bursting pacemaker potential activity. In: Chalazonitis N, Boisson M (eds) Abnormal neuronal discharges. Raven Press, New-York, pp 359–387
Bennett MVL (1972) A comparison of electrically and chemically mediated transmission. In: Pappas GG, Purpura DP (eds) Structure and function of synapses. Raven Press, New-York, pp 221–256
Burrows M, Siegler MVS (1978) Graded synaptic transmission between local interneurons and motorneurones in the metathoracic ganglion of the locust. J Physiol (Lond) 285:231–256
Carpenter DO, Gunn R (1970) The dependence of pacemaker discharge ofAplysia neurons upon Na+ and Ca++. J Cell Physiol 75:121–128
Clusin W, Spray DC, Bennett MVL (1975) Activation of a voltageinsensitive conductance by inward calcium current. Nature 256:425–427
Connor JA (1979) Calcium current in molluscan neurones: Measurement under conditions which maximize its visibility. J Physiol (Lond) 286:41–60
Connor JA, Prosser CL, Weems WA (1974) A study of pace-maker activity in intestinal smooth muscle. J Physiol (Lond) 240:671–701
Fain GL, Quandt FN, Gerschenfeld HM (1977) Calcium dependent regenerative responses in rods. Nature 269:707–710
Gadsby DC, Cranefield PF (1977) Two levels of resting potential in cardiac Purkinje fibers. J Gen Physiol 70:725–746
Gainer H (1972) Electrophysiological behavior of an endogeneously active neurosecretory cell. Brain Res 39:403–418
Gola M (1974) Neurones à ondes-salves des mollusques. Variations cycliques lentes des conductances ioniques. Pfluegers Arch 352:17–36
Gola M (1976) Electrical properties of bursting pacemaker neurones. In: Salanki J (ed) Neurobiology of invertebrates. Akademiai Kiado, Budapest, pp 381–421
Gola M (1978) A model for the production of slow potential waves and associated spiking in molluscan neurons. In: Chalazonitis N, Boisson M (eds) Abnormal neuronal discharges. Raven Press, New-York, pp 243–261
Gola M, Ducreux C, Chagneux H (1977) Ionic mechanism of slow potential wave production in barium-treatedAplysia neurons. J Physiol (Paris) 73:407–440
Gorman ALF, Thomas MV (1978) Changes in the intracellular concentration of free calcium ions in a pacemaker neurone, measured with the metallochromic indicative dye Arsenazo III. J Physiol (Lond) 275:357–376
Graubard K (1978) Synaptic transmission without action potentials: Input output properties of a non-spiking interneuron. J Neurophysiol 41:1014–1025
Grundfest H (1972) N-shaped characteristics in living membranes. In: Agin DP (ed) Perspectives in membrane biophysics. Gordon and Breach, New-York, pp 37–63
Gulrajani RM, Roberge FA (1978) Possible mechanisms underlying bursting pacemaker discharges in invertebrate neurons. Fed Proc 37:2146–2152
Hagiwara S, Naka K (1964) The initiation of spike potential in barnacle muscle fibers under low internal calcium. J Gen Physiol 48:141–162
Hagiwara S, Fukuda J, Eaton DC (1974) Membrane currents carried by Ca, Sr and Ba in barnacle muscle fiber during voltage clamp. J Gen Physiol 63:564–578
Heyer CB, Lux HD (1976) Control of the delayed outward potassium currents in bursting pacemaker neurons ofHelix pomatia. J Physiol (Lond) 262:349–382
Heyer CB, Lux HD (1978) Unusual properties of the Ca-K system responsible for prolonged action potentials in neurons from the snailHelix pomatia. In: Chalazonitis N, Boisson M (eds) Abnormal neuronal discharges. Raven Press, New York, pp 311–327
Junge D, Stephens CL (1973) Cyclic variation of potassium conductance in a burst-generating neurone ofAplysia. J Physiol (Lond) 235:155–181
Kandel ER (1976) Cellular basis of behavior. An introduction to behavioral neurobiology. Kandel ER (ed). Freeman, Reading
Krnjevic K, Pumain R, Renaud L (1971). Effect of Ba++ and tetraethylammonium on cortical neurones. J Physiol (Lond) 215:223–245
Lux HD, Eckert R (1974) Inferred slow inward current in snail neurones. Nature 250:574–576
Marmor MF (1971) The effects of temperature and ions on the current-voltage relation and electrical characteristics of a molluscan neurone. J Physiol (Lond) 218:573–598
Mathieu PA, Roberge FA (1971) Characteristics of pacemaker oscillations inAplysia neurons. Can J Physiol Pharmacol 49:787–795
Maynard DM, Selverston AI (1975) Organization of the stomatogastric ganglion of the spiny lobster. IV. The pyloric system. J Comp Physiol 100:161–182
Meech RW (1972) Intracellular calcium injection causes increased potassium conductance inAplysia nerve cells. Comp Biochem Physiol [A] 42:493–499
Meech RW (1974) The sensitivity ofHelix aspersa neurones to injected calcium ions. J Physiol (Lond) 237:259–277
Meech RW, Standen NB (1975) Potassium activation inHelix aspersa neurones under voltage clamp: A component mediated by calcium influx. J Physiol (Lond) 249:211–239
Mendelson M (1971) Oscillator neurons in crustacean ganglia. Science 171:1170–1173
Moreton RB (1968) An application of the constant-field theory to the behaviour of giant neurones of the snailHelix aspersa. J Exp Biol 48:611–624
Mounier Y, Vassort G (1975) Evidence for a transient potassium membrane current dependent on calcium influx in crab muscle fibre. J Physiol (Lond) 251:609–625
Neher E, Lux HD (1972) Differential action of TEA+ on two K+-current components of a molluscan neurone. Pflügers Arch 336:87–100
Ohba M, Sakamoto Y, Tomita T (1975) The slow wave in the circular muscle of the guinea-pig stomach. J Physiol (Lond) 253:505–516
Pearson KG, Fourtner CR (1975) Nonspiking interneurons in walking system of the cockroach. J Neurophysiol 38:33–52
Prosser CL (1978) Rhythmic potentials in intestinal muscle. Fed Proc 37:2153–2157
Raper J (1979) Non-input mediated synaptic transmission during the generation of a cyclic motor program. Science 205:304–306
Selverston A (1974) Structural and functional basis of motor pattern generation in the stomatogastric ganglion of the lobster. Am Zool 14:957–972
Selverston AI, Russel DF, Miller JP, King DG (1976) The stomatogastric nervous system; Structure and function of a small neural network. Prog Neurobiol 6:1–75
Smith TG, Barker JL, Gainer H (1975) Requirements for bursting pacemaker potential activity in molluscan neurons. Nature 253:450–452
Strumwasser F (1968) Membrane and intracellular mechanisms governing endogenous activity in neurons. In: Carlson FD (ed) Physiological and biochemical aspects of nervous integration. Englewood Cliffs, New-York, pp 329–341
Tazaki K (1971) The effects of tetrodotoxin on the slow potential and spikes in the cardiac ganglion of a crab,Eriocheir japonicus. Jpn J Physiol 21:529–536
Thompson SH (1977) Three pharmacologically distinct potassium channels in molluscan neurones. J Physiol (Lond) 265:465–488
Vassort G (1975) Voltage-clamp analysis of transmembrane ionic currents in guinea-pig myometrium: evidence for an initial potassium activation triggered by calcium influx. J Physiol (Lond) 252:713–734
Watanabe A, Obara S, Akiyama T (1967) Pacemaker potentials for the periodic burst discharge in the heart ganglion of a stomatopod,Squilla oratoria. J Gen Physiol 50:839–862
Weisblat DA, Byerly L, Russel RL (1976) Ionic mechanisms of electrical activity in somatic muscle of the NematodeAscaris lumbricoides. J Comp Physiol 111:93–113
Wilson WA, Wachtel H (1974) Negative resistance characteristic essential for the maintenance of slow oscillations in bursting neurons. Science 186:932–934
Author information
Authors and Affiliations
Additional information
This work was supported by N.I.H. grant no. 09322, NSF grant no. 00250, and a Guggenheim Foundation Fellowship to A.D.S. and by the CNRS and a DGRST grant no. 16501891 to M. Gola. We are grateful to Stuart Thompson and Felix Strumwasser for helpful comments and to Barbara McLean for technical assistance.
Rights and permissions
About this article
Cite this article
Gola, M., Selverston, A. Ionic requirements for bursting activity in lobster stomatogastric neurons. J. Comp. Physiol. 145, 191–207 (1981). https://doi.org/10.1007/BF00605033
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00605033