Abstract
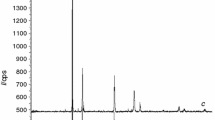
As a part of an overall study to explore the potential application of stabilized Bi2O3 as oxygen separator in various electrochemical systems, an investigation of the stability and transport characteristics of yttria- and niobia-stabilized bismuth oxide was undertaken. Polycrystalline Bi2O3 samples containing 25mol % Y2O3 were fabricated by pressureless sintering powder compacts at 1000‡ C in air. Samples containing 15mol % Nb2O5 were also fabricated by pressureless sintering at 900‡ C in air. The resulting samples were dense and of an equiaxed microstructure with grain size in the range from 28Μm for the yttria-stabilized and 42Μm for the niobia-stabilized materials, respectively. X-ray diffraction of the as-sintered specimens showed them to be single phase with CaF2-type structure. Ionic conductivity was measured by an a.c. technique over a wide range of temperatures. It was observed that the ionic conductivity of the yttria-stabilized bismuth oxide was greater than that of the niobia-stabilized one.
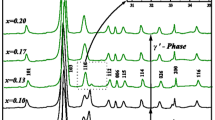
The specimens subsequently were annealed over a range of temperatures between 600‡ C and 700‡ C for up to several days. X-ray diffraction traces taken on the Y2O3-stabilized samples indicated that the original cubic solid solution had decomposed. The decomposition of the yttria-stabilized samples was also accompanied by the occurrence of exaggerated grain growth. The observed decomposition is not in agreement with the phase diagram available in the literature, according to which the cubic phase should be stable over the range of temperatures the samples were annealed in the present study. By contrast, Nb2O5-stabilized Bi2O3 remained cubic, although it appeared to have dissociated into two cubic solid solutions of slightly differing lattice parameters. There was no perceptible change in the grain size of the niobia-stabilized samples.
Several electrolyte tubes made of the yttria- and niobia-stabilized bismuth oxide were electrolytically tested under a d.c. mode with silver electrodes. In tubes made of the yttriastabilized material, the current density decreased with time (under a constant applied voltage) at 650‡ C and at ⩽ 700‡C but did not at ⩾ 700‡C consistent with the observation that the material did not decompose at ⩾ 700‡ C but did at 650‡ C. At 600‡ C, the rate of decrease was slower than at 650‡ C indicating that the kinetics of phase decomposition is probably slower at 600‡ C. In the niobia-stabilized tubes the decrease in the current density was lower. This decrease is probably related to the apparent formation of two cubic solid solutions of slightly differing compositions.
The present work shows that the published phase diagram of the Y2O3-Bi2O3 system is incorrect. The present results also suggest that for application to temperatures as low as 650‡ C (and possibly lower), electrolytes made with Nb2O5 as the stabilizer are preferable.
Similar content being viewed by others
References
G. Gattow andH. Schröder,Z. Anorg. Allg. Chem. 318 (1962) 176.
G. Gattow andD. Schultze,Z. Anorg. Alleg. Chem. 328 (1964) 44.
M. G. Hapase andV. B. Tare,Indian J. Pure Appl. Phys. 5 (1967) 401.
T. Takahashi, H. Iwahara andY. Nagai,J. Appl. Electrochem. 2 (1972) 97.
T. Takahashi andH. Iwahara,J. Appl. Electrochem. 3 (1973) 65.
R. Mansfield, in Proceedings Physics Society, London62 (1949) 476.
H. A. Harwig andA. G. Gerards,J. Solid State Chem. 26 (1978) 265–74.
T. Takahashi, H. Iwahara andT. Arao,J. Appl. Electrochem. 5 (1975) 187.
T. Takahashi, T. Esaka andH. Iwahara,J. Appl. Electrochem. 5 (1975) 197.
E. M. Levin andR. S. Roth,J. Res. Natl. Bur. Standards 68A [2] (1964) 200.
R. S. Roth andJ. Waring,J. Res. Natl. Bur. Standards 66A [6] (1962) 461.
T. Takahashi, H. Iwahara andT. Esaka,J. Electrochem. Soc. 124 [10] (1977) 1563.
T. Takahashi andH. Iwahara,Mater. Res. Bull. 13 (1978) 1447.
R. K. Datta andJ. P. Meehan,Z. Anorg. Allg. Chem. 383 [3] (1971) 328.
A. R. Cooper, Jr andJ. H. Heasley,J. Amer. Ceram. Soc. 49 [5] (1966) 280–4.
A. V. Virkar andM. R. Plichta,J. Amer. Ceram. Soc. 66 [6] (1983) 451–6.
T. C. Yuan andA. V. Virkar,J. Amer. Ceram. Soc. 71 [1] (1988) 12–22.
S. J. Kim, Z. C. Chen andA. V. Virkar,J. Amer. Ceram. Soc. 71 [10] (1988) C428-C432.
R. M. Cohen, D. Drobeck andA. V. Virkar,J. Amer. Ceram. Soc. 71 [9] (1988) C401-C403.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Joshi, A.V., Kulkarni, S., Nachlas, J. et al. Phase stability and oxygen transport characteristics of yttria- and niobia-stabilized bismuth oxide. J Mater Sci 25, 1237–1245 (1990). https://doi.org/10.1007/BF00585430
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/BF00585430