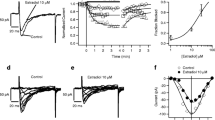
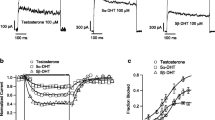
Abstract
On porcine intermediate lobe (IL) endocrine cells, spontaneously opening chloride channels have been studied and compared to GABA-A activated chloride channels. Elementary currents were recorded mainly from outside-out patches excised from IL cells maintained in culture for 1–4 weeks. Spontaneous inward currents were observed in Cs-loaded cells after replacing Na in the extracellular medium by the impermeant ion choline. This activity, at an internal calcium concentration of 10−8 M corresponded to a channel for chloride ions with a main conductance level of 26 pS, and substates around 11 pS. The sequence of permeabilities to halides was I>Br>Cl. These conductance characteristics were common to the GABA-operated channels which also showed a main conductance substate of 23–31 pS. The open time of the 26 pS level mostly encountered in spontaneous activity, was distributed along two modes: one, the most frequent, around 1 ms, and the other around 4 ms. This latter mode was the predominant one observed during GABA and isoguvacine applications but in addition a bursting activity of 19 ms duration was also seen. Specific GABA-A receptor antagonists (bicuculline and SR 42641, 1 μM) blocked activity evoked by GABA (1–10 μM), but did not affect spontaneous events. These spontaneous Cl events were only observed in a restricted range of internal Ca concentrations, i.e. between 1 nM and 0.1 μM, and were practically abolished at Cai 1 μM. The GABA-induced activity of Cl channels was also Ca-sensitive, being reduced when Cai reached 1 μM.
Similar content being viewed by others
References
Barker JL, McBurney RN, MacDonald JF (1982) Fluctuation analysis of neutral amino acid responses in cultured mouse spinal neurones. J Physiol (Lond) 322:365–387
Bormann J, Clapham DE (1985) γ-Aminobutyric acid receptor channels in adrenal chromaffin cells: a patch-clamp study. Proc Natl Acad Sci USA 82:2168–2172
Bormann J, Sakmann B, Seifert W (1983) Isolation of GABA-activated single-channel Cl− currents in the soma membrane of rat hippocampal neurones. J Physiol (Lond) 341:9P
Bowery NG, Price CG, Hudson AL, Hill DR, Wilkin GP, Turnbull MJ (1984) GABA receptor multiplicity: visualization of different receptor types in the mammalian CNS. Neuropharmacology 23:219–232
Chambon JP, Feltz P, Heaulme M, Restle S, Schlichter R, Biziere K, Wermuth CG (1985) An arylpyridazine derivative of γ-aminobutyric acid (GABA) is a selective and competitive antagonist at the GABAA receptor site. Proc Natl Acad Sci USA 82:1832–1836
Chesnoy-Marchais D, Evans MG (1984) Two types of chloride channels in outside-out patches fromAplysia neurones. J Physiol (Lond) 357:64P
Colquhoun D, Sakmann B (1981) Fluctuations in the microsecond time range of the current through single acetylcholine receptor ion channels. Nature 294:464–466
Cull-Candy SG, Miledi R (1981) Junctional and extrajunctional membrane channels activated by GABA in locust muscle fibres. Proc R Soc (LOnd) B 211:527–535
Cull-Candy SG, Ogden DC (1985) Ion channels activated byl-glutamate and GABA in cultured cerebellar neurons of the rat. Proc R Soc (Lond) B 224:367–373
Curtis DR, Malik R (1984) Ethylenediamine: a GABA mimetic? Trends Pharmac Sci 5:458–459
Demeneix BA, Desaulles E, Feltz P, Loeffler J-Ph (1984) Dual population of GABA-A and GABA-B receptors in rat pars intermedia demonstrated by release of α-MSH caused by barium ions. Br J Pharmacol 82:183–190
Demeneix BA, Feltz P, Loeffler J-Ph (1986a) GABA-ergic mechanisms and their functional relevance in the pituitary. In: Erdö SL, Bowery NG (eds) GABA-ergic mechanisms in the mammalian periphery. Raven Press, New York, pp 261–289
Demeneix BA, Taleb O, Loeffler J-Ph, Feltz P (1986b) GABA-A and GABA-B receptors on procins pars intermedia cells in primary culture: functional role in modulating peptide release. Neuroscience 17:1275–1285
Désarménien M, Desaulles E, Feltz P, Hamann M, Michaud JC, Mienville JM (1986) Electrophysiological study and pyridazine-GABA derivatives with GABA-A antagonist properties. Br J Pharmacol 87:105P
Gershengorn MC, Thaw C (1985) Thyrotropin-releasing hormone (TRH) stimulates biphasic elevation of cytoplasmic free calcium in GH3 cells. Further evidence that TRH mobilizes cellular and extracellular Ca. Endocrinology 116:591–596
Gold MR, Martin AR (1984) γ-Aminobutyric acid and glycine activate Cl channels having different characteristics in CNS neurones. Nature (Lond) 308:639–641
Gray R, Johnston D (1985) Rectification of single GABA gated chloride channels in adult hippocampal neurons. J Neurophysiol 54:134–142
Hamill OP, Bormann J, Sakmann B (1983) Activation of multipleconductance state chloride channels in spinal neurones by glycine and GABA. Nature 305:805–808
Heaulme M, Chambon JP, Leyris R, Molimard J-Ch, Wermuth CG, Biziere K (1986) Biochemical characterization of the interaction of three Pyridazinyl-GABA derivatives with the GABA-A receptor site. Brain Res 384:224–231
Hodgson AJ, Penke B, Erdei A, Chubb IW, Somogyi P (1985) Antisera to γ-aminobutyric acid. I. Production and characterization using a new model system. J Histochem Cytochem 33:22–239
Jackson MB, Lecar H, Mathers DA, Barker JL (1982) Single channel currents activated by GABA, muscimol and (−)pentobarbital in cultured mouse spinal neurons. J Neurosci 2:889–894
Kataoka Y, Gutman Y, Guidotti A, Panula P, Wroblewski Y, Cosenza-Murphy D, Wu D, Costa E (1984) Intrinsic GABA-ergic system of adrenal chromaffin cells. Proc Natl Acad Sci USA 81:3218–3222
Krishtal OA, Pidoplichko VI (1980) A receptor for protons in the nerve cell membrane. Neuroscience 5:2325–2327
Loeffler J-Ph, Demeneix BA, Pittius CW, Kley N, Haegele KD, Höllt V (1987) GABA differentially regulates the gene expression of proopiomelanocortin in rat intermediate and anterior pituitary. Peptides 7:253–258
Martin RJ (1985) γ-aminobutyric acid and piperazine-activated single channel currents formAscaris suum body muscle. Br J Pharmac 84:445–463
Marty A, Tan YP, Trautmann A (1984) Three types of calciumdependent channel in rat lacrimal glands. J Physiol (Lond) 357:293–325
Marty A, Evans MG, Tan YP, Trautmann A (1986) Muscarinic response in rat lacrimal glands. J Exp Biol 124:15–32
Mathers DA (1985) Spontaneous and GABA-induced single channel currents in cultured murine spinal cord neurons. Can J Physiol Pharmacol 63:1228–1233
Mayer ML (1985) A calcium-activated chloride current generates the after-depolarization of rat sensory neurones in culture. J Physiol (Lond) 364:217–239
McBurney RN, Smith SM, Zorec R (1985) Conductance states of γ-aminobutyric acid (GABA)- and glycine-activated chloride (Cl−) channels in rat spinal neurones in cell culture. J Physiol (Lond) 365:87P
Neering IR, McBurney RN (1984) Role of microsomal Ca store in mammalian neurones? Nature 309:158–160
Oertel WH, Mugnaini E, Tappaz ML, Weise VK, Dahl AJ, Schemchel DE, Kopin IJ (1982) Central GABAergic innervation of neurointermediate pituitary lobe, biochemical and immunocytochemical study in the rat. Proc Natl Sci USA 79:675–679
Owen DA, Segal M, Barker JL (1984) A Ca-dependent Cl conductance in cultured spinal cord neurones. Nature 311:567–570
Ozawa S, Yuzaki M (1984) Patch-clamp studies of chloride channels activated by gamma-aminobutyric acid in cultured hippocampal neurones of the rat. Neurosci Res 1:275–293
Segal M, Barker JL (1984) Rat hippocampal neurons in culture: properties of GABA-activated Cl ion conductance. J Neurophysiol 51:500–515
Sigworth F (1983) An example of analysis. In: Sakmann B, Neher E (eds) Single channel recording. Plenum Press, New York London, pp 301–321
Stephenson FA, Casalotti SO, Mamalaki C, Barnard EA (1986) Antibodies recognising the GABAA/Benzodiazepine receptor including its regulatory sites. J Neurochem 46:963–967
Stoeckel ME, Schmitt G, Porte A (1981) Fine structure and cytochemistry of the intermediate lobe of the mammalian pituitary gland under normal and experimental conditions. In: Ciba Foundation Symposium. Peptides of the pars intermedia. Pittman Medical. London, pp 101–127
Taleb O, Loeffler J-Ph, Trouslard J, Demeneix BA, Kley N, Höllt V, Feltz P (1987) Ionic conductances related to GABA action on secretory and biosynthetic activity of pars intermedia cells. Brain Res Bull 17:725–730
Taraskevich PS, Douglas WW (1982) GABA directly affects electrophysiological properties of pituitary pars intermedia cells. Nature 299:733–734
Taraskevich PS, Douglas WW (1985) Pharmacological and ionic features of γ-aminobutyric acid receptors influencing electrical properties of melanotrophs isolated from the rat pars intermedia. Neuroscience 14:301–308
Tilders FJH, Berkenbosch F, Smelik PJ (1985) Control of secretion of peptides related to adrenocorticotropin, melanocyte stimulating hormone and endorphin. From Horm Res 14:161–196
Tomiko SA, Taraskevich PS, Douglas WW (1983) GABA acts directly on cells of pituitary pars intermedia to alter hormonal output. Nature 301:706–707
Tunnicliff G, Smith JA (1981) Competitive inhibition of γ-aminobutyric acid receptor binding by N-2-hydroethyl-piperazine-N′-2-ethanesulfonic acid and related buffers. J Neurochem 36:1222–1226
Vincent SR, Hökfelt T, Wu JY (1982) GABA neuron systems in hypothalamus and pituitary gland. Immunohistochemical demonstration using antibodies against glutamate decarboxylase. Neuroendocrinology 34:117–125
Yasui S, Ishizuka S, Akaike N (1985) GABA activates different types of chloride-conducting receptor-ionophore complexes in a dose-dependent manner. Brain Res 344:176–180
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Taleb, O., Trouslard, J., Demeneix, B.A. et al. Spontaneous and GABA-evoked chloride channels on pituitary intermediate lobe cells and their internal Ca requirements. Pflugers Arch. 409, 620–631 (1987). https://doi.org/10.1007/BF00584663
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00584663