Abstract
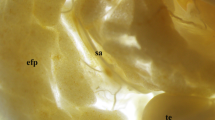
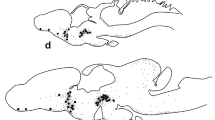
The presence and distribution of peptidergic nerve fibers were studied in the testis and mesorchium of the toad by means of immunohistochemistry. Cryostat sections of the testis and whole-mount preparations of mesorchia were immunostained with antisera to calcitonin gene-related peptide (CGRP) and neuropeptide tyrosine (NPY). After leaving the mesorchium CGRP-immunoreactive (IR) fibers were seen predominantly running in between the seminiferous tubules. In addition, a small population of CGRP-IR nerve fibers formed thin plexuses around blood vessels. Conversely, NPY-like immunoreactivity predominated in nerve fibers that formed dense plexuses around vessels both in the mesorchium and testis. Additionally, some single NPY-IR nerve fibers could be seen in both structures studied. The functional significance of these peptidergic systems in the testis of the toad remains to be analyzed.
Similar content being viewed by others
References
Adrian TE, Gu J, Allen JM, Tatemoto K, Polak JM, Bloom SR (1984) Neuropeptide Y in the human male genital tract. Life Sciences 35:2643–2648
Alm P, Alumets J, Brodin E, Håkanson R, Nilsson G, Sjöberg NO, Sundler F (1978) Peptidergic (substance P) nerves in the genito-urinary tract. Neuroscience 3:419–425
Alm P, Alumets J, Håkanson R, Owman C, Sjöberg N-O, Sundler F, Walles B (1980) Origin and distribution of VIP (vasoactive intestinal polypeptide)-nerves in the genito-urinary tract. Cell Tissue Res 205:337–347
Bell C, McLean JR (1973) The autonomic innervation of the rat testicular capsule. J Reprod Fertil 32:253–258
Campos MB, Ritta MN (1993) Effect of bilateral denervation of the immature rat testis on testicular gonadotropin receptors and in vitro androgen production. Neuroendocrinology 57:189–194
Costa M, Buffa R, Furness JB, Solcia E (1980) Immunohistochemical localization of polypeptides in peripheral autonomic nerves using whole mount preparations. Histochemistry 65:157–165
Edvinson L, Emson P, McCulloch J, Tatemoto K, Uddman R (1983) Neuropeptide Y: Cerebrovascular innervation and vasomotor effects in the cat. Neurosci Lett 43:79–84
Fahrenkrug J, Palle C, Jorgensen J, Otteson B (1989) Regulatory peptides in the mammalian urogenital system. In: Polak JM (ed) Regulatory peptides. Birkhäuser, Basel, pp 362–381
Frankel AI, Chapman JC (1984) Hypophysectomy and hemivasectomy can inhibit the testicular hemicastration response of the mature rat. Biol Reprod 30:804–808
Frankel AI, Ryan EL (1981) Testicular innervation is necessary for the response of plasma testosterone levels to acute stress. Biol Reprod 24:491–495
Gresik EW (1973) Fine structural evidence for the presence of nerve terminals in the testis of a teleost,Oryzias latipes. Gen Comp Endocrinol 21:210–213
Gu J, Polak JM, Probert L, Islam KN, Marangos PJ, Mina S, Adrian TE, McGregor GP, O'Shaughnessy DJ, Bloom SR (1983) Peptidergic innervation of the human male genital tract. J Urol 130:386–391
Horn JP, Stofer WD (1988) Double labeling of the paravertebral sympathetic C system in the bullfrog with antisera to LHRH and NPY. J Auton Nerv Syst 23:17–24
Horn JP, Stofer WD, Fatherazi S (1987) Neuropeptide Y-like immunoreactivity in bullfrog sympathetic ganglia is restricted to C cells. J Neurosci 7:1717–1727
Jan LI, Jan YN (1982) Peptidergic trasmission in sympathetic ganglia of the frog. J Physiol 327:219–246
Jan LI, Jan NY, Brownfield MS (1980) Peptidergic transmitters in synaptic boutons of sympathetic ganglia. Nature 288:380–382
Johnson DG, De Nogueira Araujo GM (1981) A simple method of reducing the fading of immunofluorescence during microscopy. J Immunol Methods 43: 349–350
Kuramoto H (1987) An immunohistochemical study of chromaffin cells and nerve fibers in the adrenal gland of the bullfrog,Rana catesbiana. Arch Histol Jap 50:15–38
Larsson LI, Fahrenkrug J, Schaffalitzky de Muckadell OB (1977) Occurrence of nerves containing vasoactive intestinal polypeptide immunoreactivity in the male genital tract. Life Sciences 21:503–508
Lundberg JM, Terenius L, Hökfelt T, Martling CR, Tatemoto K, Mutt V, Polak J, Bloom S, Goldstein M (1982) Neuropeptide Y (NPY)-like immunoreactivity in peripheral noradrenergic neurons and effects of NPY on sympathetic function. Acta Physiol Scand 116:477–480
Morris JL, Gibbins IL, Campbell G, Murphy R, Furness JB, Costa M (1986) Innervation of the large arteries and heart of the toad (Bufo marinus) by adrenergic and peptide-containing neurons. Cell Tissue Res 243:171–184
Properzi G, Cordeschi G, Francavilla S (1992) Postnatal development and distribution of peptide-containing nerves in the genital system of the male rat. Histochemistry 97:61–68
Rosenfeld MG, Mermod J-J, Amara SG, Swanson LW, Sawchenko PE, Rivier J, Vale WW, Evans RM (1983) Production of a novel neuropeptide encoded by the calcitonin gene via tissue-specific RNA processing. Nature 304:129–135
Schindelmeiser J, Bergmann M, Straub H, Greven H (1983) Morphological and biochemical studies on the innervation of the testis ofSalamandra salamandra L. (Amphibia, Urodela). J Auton Nerv Syst 8:65–78
Setchell BP, Maddocks S, Brooks DE (1994) Anatomy, vasculature, innervation, and fluids of the male reproductive tract. In: Knobil E, Neill J et al. (eds) The Physiology of Reproduction. Raven Press, New York, pp 1063–1176
Shu S, Ju G, Fan L (1988) The glucose oxidase method in peroxidase histochemistry of the nervous system. Neurosci Lett 85:169–171
Unsicker K (1973) Innervation of the testicular interstitial tissue in reptiles. Z Zellforsch 146:123–138
Unsicker K, Azelsson S, Owman C, Svensson KG (1975) Innervation of the male genital tract and kidney in the Amphibia,Xenopus laevis Daudin,Rana temporaria L., andBufo bufo L. Cell Tissue Res 160:453–484
Valasti A, Linnoila I, Hervonen A (1980) Immunohistochemical demonstration of VIP, (met)- and (leu)-enkephalin immunoreactive nerve fibers in the human prostate and seminal vesicles. Histochemistry 66:89
Zamboni L, De Martino C (1967) Buffered picric acid formaldehyde: a new rapid fixation for electron microscopy. J Cell Biol 35:148A
Zhu BC, Chiocchio SR, Suburo AM, Tramezzani JH (1995) Contribution of the superior and the inferior spermatic nerves to the innervation of the testis in the rat. J Androl (in press)
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Achi, M.V., Figueroa, J.M., Nicolini, V.G. et al. NPY- and CGRP-like immunoreactive nerve fibers in the testis and mesorchium of the toad (Bufo arenarum). Cell Tissue Res. 281, 375–378 (1995). https://doi.org/10.1007/BF00583406
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00583406