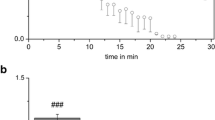
Abstract
In rat lacrimal gland cells, application of acetylcholine (ACh) opens Ca-dependent channels and closes gap junction channels. We have shown previously that the increase in intracellular calcium concentration induced by ACh, is not required for the closure of gap junctions [20]. We have examined the effects of activators of protein kinase C on gap junction conductance measured with the double patch-clamp technique. This conductance was markedly reduced by incubating the cell pairs for a few minutes with 100 nM phorbol dibutyrate (PdBu). Two membrane permeant analogues of diacylglycerol, OAG (1-oleoyl-2-acetyl-glycerol) and DOG (1,2-dioleoyl-glycerol) also induced a closure of gap junction channels. This effect was observed in the concentration range 10–100 μM when the diacylglycerol was added on intact cells, and at 75μM when it was applied on dialysed cells. The cell uncoupling was not mediated by phosphatidate, a degradation product of OAG, nor by a phospholipase A2-induced increase in arachidonate concentration. The OAG-induced closure of gap junction channels reversed spontaneously upon prolonged exposure (more than 90 min at 37°C) to 25 μM OAG. After a prolonged OAG treatment, the ability of ACh to uncouple the cells was markedly reduced. ACh induced uncoupling was modulated to some extent by intracellular Ca and had an absolute requirement for Mg. These results indicate that ACh-induced closure of gap junction channels may be mediated by PKC.
Similar content being viewed by others
References
Davidson JS, Baumgarten IM, Harley EH (1985) Studies on the mechanism of phorbol ester-induced inhibition of intracellular junctional communication. Carcinogenesis 6:1353–1358
Dawson RMC, Irvine RF, Bray J, Quinn PJ (1984) Long-chain unsaturated diacylglycerols cause a perturbation in the structure of phospholipid bilayers rendering them susceptible to a phospholipase attack. Biochem Biophys Res Commun 125:836–842
Enomoto T, Yamasaki H (1985) Rapid inhibition of intercellular communication between BALB/c3T3 cells by diacylglycerol, a possible endogenous functional analogue of phorbol esters. Cancer Res 45:3706–3710
Enomoto T, Sasaki Y, Shiba Y, Kanno Y, Yamasaki H (1981) Tumor promoters cause a rapid and reversible inhibition of the formation and maintenance of electrical cell coupling in culture. Proc Natl Acad Sci USA 78:5628–5632
Evans MG, Marty A (1986) Potentiation of muscarinic and α-adrenergic responses by an analogue of guanosine 5′-triphosphate. Proc Natl Acad Sci USA 83:4099–4103
Evans MG, Marty A (1986) Calcium-dependent chloride currents in isolated cells from rat lacrimal glands. J Physiol 378:437–460
Fitzgerald DJ, Knowles SE, Ballard FJ, Murray AW (1983) Rapid and reversible inhibition of junctional communication by tumor promoters in a mouse cell line. Cancer Res 43:3614–3618
Ginsborg BL, House CR (1980) Stimulus-response coupling in gland cells. Annu Rev Biophys Bioeng 9:55–80
Hidaka H, Hagiwara M (1987) Pharmacology of the isoquinoline sulfonamide protein kinase C inhibitors. TIPS 8:162–164
Krishtal OA, Pidoplichko VI (1980) A receptor for protons in the nerve cell membrane. Neuroscience 5:2325–2327
Liano I, Marty A (1987) Protein kinase C activators inhibit the inositoltrisphosphate-mediated muscarinic current response in rat lacrimal glands. J Physiol 394:239–248
Loewenstein WR (1985) Regulation of cell-to-cell communication by phosphorylation. Biochem Soc Symp 50:43–58
Marty A, Neher E (1983) Tight-seal whole-cell recording: In: Sakmann B, Neher E (eds) Single-channel recording. Plenum Press, New York, pp 107–122
Marty A, Evans MG, Tan YP, Trautmann A (1986) Muscarinic response in rat lacrimal glands. J Exp Biol 124:15–32
Moolenaar WH, Kruijer W, Tilly BC, Verlaan I, Bierman AJ, de Laat SW (1986) Growth factor-like action of phosphatidic acid. Nature 323:171–173
Mori T, Takai Y, Minakuchi R, Yu B, Nishizuka Y (1980) Inhibitory action of chlorpromazine, dibucaine, and other phospholipids-interacting drugs on calcium-activated, phospholipid-dependent protein kinase. J Biol Chem 255:8378–8380
Murray AW, Fitzgerald DJ (1979) Tumor promoters inhibit metabolic cooperation in cocultures of epidermal 3T3 cells. Biochem Biophys Res Commun 91:395–401
Neyton J, Trautmann A (1985) Single-channel currents of an intercellular junction. Nature 317:331–335
Neyton J, Trautmann A (1986a) Physiological modulation of gap junction permeability. J Exp Biol 124:93–114
Neyton J, Trautmann A (1986b) Acetylcholine modulation of the conductance of intercellular junctions between rat lacrimal cells. J Physiol 377:283–295
Nishizuka Y (1984) The role of protein kinase C in cell surface signal transduction and tumour promotion. Nature 308:693–698
Nishizuka Y (1986) Studies and perspectives of protein kinase C. Science 233:305–312
Petersen OH (1980) The electrophysiology of gland cells. Academic Press, London
Trautmann A, Marty A (1984) Activation of Ca-dependent K channels by carbamylcholine in rat lacrimal glands. Proc Natl Acad Sci USA 81:611–615
Trautmann A, Neyton J, Randriamampita C, Giaume C (1988) Role of divalent cations in the control of gap junction conductance in a rat exocrine gland. In: Gap junctions. Alan Liss Inc (in press)
Yada T, Rose B, Loewenstein WR (1905) Diacylglycerol downregulates junctional membrane permeability. TMB-8 blocks this effect. J Membr Biol 88:217–232
Yamasaki H, Enomoto T, Martel N, Shiba Y, Kanno Y (1983) Tumor promoter-mediated reversible inhibition of cell-cell communication (electrical coupling); relationship with phorbol ester binding and de novo macromolecule synthesis. Exp Cell Res 146:297–308
Yancey SB, Edens JE, Trosko JE, Chang CC, Revel JP (1982) Decreased incidence of gap junctions between chinese hamster V-79 cells upon exposure to the tumor promoter 12-O-teiradecanoyl phorbol acetate. Exp Cell Res 139:329–340
Yotti LP, Chang CC, Trosko JE (1979) Elimination of metabolic cooperation in chinese hamser cells by a tumor promoter. Science 206:1089–1091
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Randriamampita, C., Giaume, C., Neyton, J. et al. Acetylcholine-induced closure of gap junction channels in rat lacrimal glands is probably mediated by protein kinase C. Pflugers Arch. 412, 462–468 (1988). https://doi.org/10.1007/BF00582534
Received:
Revised:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00582534