Abstract
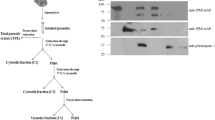
We describe antigens of Plasmodium falciparum recognised by murine monoclonal antibodies which by immunofluorescence react with the rhoptry organelles of the extracellular merozoite stage. Immunoblotting shows that the antibodies recognise two major parasite antigens of Mr 82 and 65 kilodaltons (kDa). Immunoprecipitations from detergent extracts of [35S]-methioninelabelled parasites show that the 82-kDa and 65-kDa antigens are parasite proteins. Pulse-chase experiments on synchronous parasite cultures show that the 82-kDa protein is synthesised during early schizogony and is later processed into the 65-kDa antigen in segmenting schizonts. In Nonidet P-40, these antigens are non-covalently associated with two other proteins of 40 kDa and 42 kDa. The 40/42-kDa doublet is synthesised in parallel with the 82 kDa antigen and persists, apparently unchanged, till the end of the cell cycle.
Similar content being viewed by others
Abbreviations
- HEPES :
-
N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid
- EDTA :
-
ethylenediaminetetraacetic acid
- EGTA :
-
ethyleneglycolbis-(aminoethylether) tetraacetic acid
- PMSF :
-
phenylmethylsulphonylfluoride
- TLCK :
-
tosyl-L-lysine chloromethyl ketone
- SDS-PAGE :
-
sodium dodecyl sulphate polyacrylamide gel electrophoresis
- M r :
-
relative molecular mass
References
Aikawa M (1971) Fine structure of malaria parasites. Exp Parasitol 30:284–320
Bannister LH (1977) The invasion of red cells by Plasmodium. In: Taylor AER, Mullen R (eds) Symposia of the British Society for Parasitology, vol 15. Blackwell, Oxford, pp 27–55
Bannister LH, Mitchell GH, Butcher GA, Dennis ED (1986) Lamellar membranes associated with rhoptries in erytrocytic merozoites of Plasmodium knowlesi: a clue to the mechanism of invasion. Parasitology 92:291–303
Braun-Breton C, Jendoubi M, Brunet E, Perrin L, Scaife J, Pereira da Silva L (1986) In vivo time course of synthesis and processing of major schizont membrane polypeptides in Plasmodium falciparum. Mol Biochem Parasitol 20:33–43
Campbell GH, Miller LH, Hudson D, Franco EL, Andrysiak PM (1984) Monoclonal antibody characterization of Plasmodium falciparum antigens. Am J Trop Med Hyg 33:1051–1054
David PH, Hadley TJ, Aikawa M, Miller LH (1984) Processing of a major parasite surface glycoprotein during the ultimate stages of differentiation in Plasmodium knowlesi. Mol Biochem Parasitol 11:267–282
Dvorak JA, Miller LH, Whitehouse WC, Shiroishi T (1975) Invasion of erythrocytes by malaria merozoites. Science 187:748–750
Freeman RR, Holder AA (1983) Surface antigens of malaria merozoites: a high molecular weight precursor in processed to an 83,000 molecular weight form expressed on the surface of Plasmodium falciparum merozoites. J Exp Med 158:1647–1653
Hall R, McBride JS, Morgan G, Tait A, Zolg W, Walliker D, Scaife JG (1983) Antigens of the erythrocytic stages of the human malaria parasite Plasmodium falciparum detected by monoclonal antibodies. Mol Biochem Parasitol 7:247–265
Hawkes R (1982) Identification of concavalin A-binding proteins after sodium dodecyl sulfate-gel electrophoresis and protein blotting. Anal Biochem 123:143–146
Heidrich HG (1986) Plasmodium falciparum antigens as target molecules for a protective immunization against malaria: an up-to-date review. Z Parasitenkd 72:1–11
Holder AA, Freeman RR (1981) Immunization against bloodstage rodent malaria using purified parasite antigens. Nature 294:361–364
Holder AA, Freeman RR (1982) Biosynthesis and processing of Plasmodium falciparum schizont antigen recognised by immune serum and a monoclonal antibody. J Exp Med 156:1528–1538
Holder AA, Freeman RR (1984) The three major antigens on the surface of Plasmodium falciparum are derived from a single high molecular weight precursor. J Exp Med 160:624–629
Holder AA, Freeman RR, Uni S, Aikawa M (1985) Isolation of a Plasmodium falciparum rhoptry protein. Mol Biochem Parasitol 14:293–303
Howard RF, Reese RT (1984) Synthesis of merozoite proteins and glycoproteins during the schizony of Plasmodium falciparum. Mol Biochem Parasitol 10:319–334
Howard RF, Stanley HA, Campbell GH, Reese RT (1984) Proteins responsible for a punctate fluorescence pattern in Plasmodium falciparum merozoites. Am J Trop Med Hyg 33:1055–1059
Ladda RL, Aikawa M, Sprinz H (1969) Penetration of erythrocytes by merozoites of mammalian and avian malarial parasites. J Parasitol 55:633–644
Laemmli UK (1970) Cleavage of structural proteins during assembly of the head of bacteriophage T4. Nature 277:680–682
Lambros C, Vanderberg JP (1979) Synchronization of Plasmodium falciparum erythrocyte stages in culture. J Parasitol 65:418–420
McBride JS, Walliker D, Morgan G (1982) Antigenic diversity in the human malaria parasite Plasmodium falciparum. Science 217:254–257
McBride JS, Welsby PD, Walliker D (1984) Serotyping Plasmodium falciparum from acute human infections using monoclonal antibodies. Trans R Soc Trop Med Hyg 78:32–34
McBride JS, Newbold CI, Anand R (1985) Polymorphism of a high molecular weight schizont antigen of the human malaria parasite Plasmodium falciparum. J Exp Med 161:160–180
Perrin LH, Dayal R (1982) Immunity to asexual erythrocytic stages of Plasmodium falciparum: role of defined antigens in the humoral response. Immunol Rev 61:245–269
Perrin LH, Merkli B, Gabra MS, Stocker JW, Chizzolini C, Richle R (1985) Immunization with a Plasmodium falciparum merozoite antigen induces a partial immunity in monkeys. J Clin Invest 75:1718–1721
Schofield L, Bushell GR, Cooper JA, Saul AJ, Upcroft JA, Kidson C (1986) A rhoptry antigen of Plasmodium falciparum contains conserved and variable epitopes recognised by inhibitory monoclonal antibodies. Mol Biochem Parasitol 18:183–195
Simmons DL, Hyde JE, MacKay M, Goman M, Scaife J (1985) Cloning studies on the gene coding for the L-(+)-lactate dehydrogenase of Plasmodium falciparum. Mol Biochem Parasitol 15:231–243
Thaithong S, Beale GH, Fenton B, McBride JS, Rosario V, Walker A, Walliker D (1984) Clonal diversity in a single isolate of the malaria parasite Plasmodium falciparum. Trans R Soc Trop Med Hyg 78:242–245
Trager W, Jensen JB (1976) Human malaria parasites in continuous culture. Science 193:673–675
WHO (1984) Asexual blood stage and transmission-blocking antigens of Plasmodia. Report of the 6th Meeting of the Scientific Working Group on the immunology of Malaria, Geneva, Switzerland
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Clark, J.T., Anand, R., Akoglu, T. et al. Identification and characterisation of proteins associated with the rhoptry organelles of Plasmodium falciparum merozoites. Parasitol Res 73, 425–434 (1987). https://doi.org/10.1007/BF00538200
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00538200