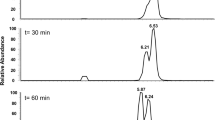
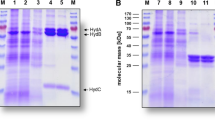
Abstract
Desulfotomaculum acetoxidans oxidizes acetate to CO2 with sulfate. This organism metabolizes acetate via a pathway in which C1 units rather than tri- and dicarboxylic acids are intermediates. We report here that cell extracts of D. acetoxidans catalyzed an exchange between CO2 and the carboxyl group of acetate at a rate of 90 nmol · min-1 · mg-1 protein which is sufficient to account for the in vivo acetate oxidation rate of 250 nmol · min-1 · mg-1 protein. The reaction was strictly dependent on both ATP and coenzyme A. The extracts contain high activities of acetate kinase (6.3 U · mg-1 protein) and phosphotransacetylase (60 U · mg-1 protein). These findings indicate that acetyl-CoA rather than acetyl-phosphate or acetate is the substrate of the carbon-carbon cleavage activity. Exchange was only observed in the presence of strong reducing agents such as Ti3+. Interestingly, the cell extracts also catalyzed the reduction of CO2 to CO with Ti3+ as electron donor (120 nmol · min-1 · mg-1 protein). Carbon monoxide dehydrogenase and other oxidoreductases involved in acetate oxidation were found to be partially associated with the membrane fraction suggesting a membrane localization of these enzymes.
Similar content being viewed by others
Abbreviations
- MOPS:
-
Morpholinopropane sulfonic acid
- Tricine:
-
N-tris(hydroxymethyl)-methylglycine
- DTT:
-
d,l-1,4-Dithiothreitol
- DMN:
-
2,3-Dimethyl-1,4-naphthoquinone
- MVOX :
-
Methyl viologen, oxidized
- APS:
-
Adenosinephosphosulfate
- SRB:
-
Sulfate reducing bacteria
- U:
-
μmol product formed per min
References
Bobik TA, Wolfe RS (1989) Activation of formylmethanofuran synthesis in cell extracts of Methanobacterium thermoautotrophicum. J Bacteriol 171:1423–1427
Bott M, Thauer RK (1987) Proton-motive-force-driven formation of CO from CO2 and H2 in methanogenic bacteria. Eur J Biochem 168:407–412
Bott M, Thauer RK (1989) The active species “CO2” formed by carbon monoxide dehydrogenase from Peptostreptococcus productus. Z Naturforsch (in press)
Bott MH, Eikmanns B, Thauer RK (1985) Defective formation and/or utilization of carbon monoxide in H2/CO2 fermenting methanogens dependent on acetate as carbon source. Arch Microbiol 143:266–269
Bott M, Eikmanns B, Thauer RK (1986) Coupling of carbon monoxide oxidation to CO2 and H2 with the phosphorylation of ADP in acetate-grown Methanosarcina barkeri. Eur J Biochem 159:393–398
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Collins MD, Widdel F (1986) Respiratory quinones of sulphatereducing and sulfur-reducing bacteria: A systematic investigation. Syst Appl Microbiol 8:8–18
Conrad R, Thauer RK (1983) Carbon monoxide production by Methanobacterium thermoautotrophicum. FEMS Microbiol Lett 20:229–232
Diekert G, Schrader E, Harder W (1986) Energetics of CO formation and CO oxidation in cell suspensions of Acetobacterium woodii. Arch Microbiol 144:386–392
Dorn M, Andreesen JR, Gottschalk G (1978) Fermentation of fumarate and l-malate by Clostridium formicoaceticum. J Bacteriol 133:26–32
Eikmanns B, Thauer RK (1984) Catalysis of an isotopic exchange between CO2 and the carboxyl group of acetate by Methanosarcina barkeri grown on acetate. Arch Microbiol 138:365–370
Eikmanns B, Fuchs G, Thauer RK (1985) Formation of carbon monoxide from CO2 and H2 by Methanobacterium thermoautotrophicum. Eur J Biochem 146:149–154
Ellermann J, Thauer RK, Bokranz M, Klein A, Voges M, Bergkessel A (1989) Methyl-coenzyme-M reductase from Methanobacterium thermoautotrophicum (strain Marburg): Purity — activity — novel inhibitors. Eur J Biochem (in press)
Fischer R, Thauer RK (1988) Methane formation from acetyl phosphate in cell extracts of Methanosarcina barkeri: dependence of the reaction on coenzyme A. FEBS Lett 228:249–253
Fischer R, Thauer RK (1989) Methyltetrahydromethanopterin as an intermediate in methanogenesis from acetate in Methanosarcina barkeri. Arch Microbiol (in press)
Fuchs G (1986) CO2 fixation in acetogenic bacteria: variations on a theme. FEMS Microbiol Rev 39:181–213
Fuchs G, Stupperich E, Eden G (1980) Autotrophic CO2 fixation in Chlorobium limicola. Evidence for the operation of a reductive tricarboxylic acid cycle in growing cells. Arch Microbiol 128:64–71
Hu SI, Drake HL, Wood HG (1982) Synthesis of acetyl coenzyme A from carbon monoxide, methyltetrahydrofolate, and coenzyme A by enzymes from Clostridium thermoaceticum. J Bacteriol 149:440–448
Hugenholtz J, Ivey DM, Ljungdahl LG (1987) Carbon monoxidedriven electron transport in Clostridium thermoautotrophicum membranes. J Bacteriol 169:5845–5847
Kengen SWM, Mosterd JJ, Nelissen RLH, Keltjens JT, van der Drift C, Vogels GD (1988) Reductive activation of the methyltetrahydromethanopterin: coenzyme M methyltransferase from Methanobacterium thermoautotrophicum strain ΔH. Arch Microbiol 150:405–412
Krone UE, Thauer RK, Hogenkamp HPC (1989) The reductive dehalogenation of chlorinated C1-hydrocarbons mediated by corrinoids. Biochemistry (in press)
Länge S, Scholtz R, Fuchs G (1989) Oxidative and reductive acetyl CoA/carbon monoxide dehydrogenase pathway in Desulfobacterium autotrophicum. 1. Characterization and metabolic function of the cellular tetrahydropterin. Arch Microbiol 151:77–83
Lee MJ, Zinder SH (1988) Isolation and characterization of a thermophilic bacterium which oxidizes acetate in syntrophic association with a methanogen and which grows acetogenically on H2−CO2. Appl Environ Microbiol 54:124–129
Ljungdahl LG (1986) The autotrophic pathway of acetate synthesis in acetogenic bacteria. Annu Rev Microbiol 40:415–450
Otto R, Lageveen G, Veldkamp H, Konings WN (1982) Lactate efflux-induced electrical potential in membrane vesicles of Streptococcus cremoris. J Bacteriol 149:733–738
Pezacka E, Wood HG (1984a) Role of carbon monoxide dehydrogenase in the autotrophic pathway used by acetogenic bacteria. Proc Natl Acad Sci USA 81:6261–6265
Pezacka E, Wood HG (1984b) The synthesis of acetyl-CoA by Clostridium thermoaceticum from carbon dioxide, hydrogen, coenzyme A and methyltetrahydrofolate. Arch. Microbiol 137:63–69
Pezacka E, Wood HG (1986) The autotrophic pathway of acetogenic bacteria. Role of CO dehydrogenase disulfide reductase. J Biol Chem 261:1609–1615
Ragsdale SW, Wood HG (1985) Acetate biosynthesis by acetogenic bacteria. Evidence that carbon monoxide dehydrogenase is the condensing enzyme that catalyzes the final step of the synthesis. J Biol Chem 260:3970–3977
Raybuck SA, Bastian NR, Orme-Johnson WH, Walsh CT (1988) Kinetic characterization of the carbon monoxide-acetyl-CoA (carbonyl group) exchange activity of the acetyl-CoA synthesizing CO dehydrogenase from Clostridium thermoaceticum. Biochemistry 27:7698–7702
Rouvière PE, Bobik TA, Wolfe RS (1988) Reductive activation of the Methyl Coenzyme M Methylreductase system of Methanobacterium thermoautotrophicum ΔH. J Bacteriol 170:3946–3952
Schauder R, Eikmanns B, Thauer RK, Widdel F, Fuchs G (1986) Acetate oxidation to CO2 in anaerobic bacteria via a novel pathway not involving reactions of the citric acid cycle. Arch Microbiol 145:162–172
Schauder R, Preuß A, Jetten M, Fuchs G (1989) Oxidative and reductive acetyl CoA/carbon monoxide dehydrogenase pathway in Desulfobacterium autotrophicum. 2. Demonstration of the enzymes of the pathway and comparison of CO dehydrogenase. Arch Microbiol 151:84–89
Simon H, Floss HG (1967) Bestimmung der Isotopenverteilung in markierten Verbindungen. Springer, Berlin Heidelberg New York
Spormann AM, Thauer RK (1988) Anaerobic acetate oxidation to CO2 in Desulfotomaculum acetoxidans. Demonstration of enzymes required for the operation of an oxidative acetyl-CoA/ carbon monoxide dehydrogenase pathway. Arch Microbiol 150:374–380
Thauer RK (1988a) Citric-acid cycle, 50 years on: Modifications and an alternative pathway in anaerobic bacteria. Eur J Biochem 176:497–508
Thauer RK (1989) Energy metabolism of sulfate reducing bacteria. In: Schlegel HG, Bowien B (eds) Biology of autotrophic bacteria, chapter 21. Science Tech Publishers, Madison, USA (in press)
Thauer RK, Rupprecht E, Jungermann K (1970) Separation of 14C-formate from CO2 fixation metabolites by isoionic-exchange chromatography. Anal Biochem 88:461–468
Thauer RK, Möller-Zinkhan D, Spormann AM (1989) Biochemistry of acetate catabolism in anaerobic chemotrophic bacteria. Ann Rev Microbiol 43:43–67
Widdel F, Pfennig N (1977) A new anaerobic, sporing, acetateoxidizing, sulfate-reducing bacterium, Desulfotomaculum (emend.) acetoxidans. Arch Microbiol 112:119–122
Widdel F, Pfennig N (1981) Sporulation and further nutritional characteristics of Desulfotomaculum acetoxidans. Arch Microbiol 129:401–402
Wood HG, Ragsdale SW, Pezacka E (1986) The acetyl-CoA pathway of autotrophic growth. FEMS Microbiol Rev 39:345–362
Yagi T, Tamiya N (1962) Enzymic oxidation of carbon monoxide. III. Reversibility. Biochim Biophys Acta 65:508–509
Zehnder AJB, Wuhrmann K (1976) Titanium(III) citrate as a nontoxic oxidation-reduction buffering system for the culture of obligate anaerobes. Science 194:1165–1166
Zinder SH, Koch M (1984) Non-aceticlastic methanogenesis from acetate: acetate oxidation by a thermophilic syntrophic coculture. Arch Microbiol 138:263–272
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Spormann, A.M., Thauer, R.K. Anaerobic acetate oxidation to CO2 by Desulfotomaculum acetoxidans . Arch. Microbiol. 152, 189–195 (1989). https://doi.org/10.1007/BF00456100
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00456100