Abstract
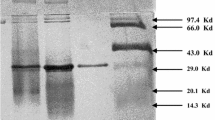
β-Ketothiolase from Zoogloea ramigera I-16-M was purified 140-fold to electrophoretic homogeneity. The bacterium appeared to contain a single isoenzyme of β-ketothiolase with a molecular weight of 190000, as determined by Sephadex G-200 gel filtration. The monomer molecular weight was 44000, as estimated by polyacrylamide gel electrophoresis in the presence of sodium dodecyl sulfate. The native enzyme thus appeared to be a tetramer with identical subunits.
The enzyme showed a pH optimum of 7.5 in the condensation reaction, and 8.5 in the thiolysis reaction. The enzyme employed a Bi Bi ping pong mechanism for the forward thiolysis reaction. The apparent K m value for acetoacetyl coenzyme A in the thiolysis reaction was 10 μM, and that for coenzyme A was 8.5 μM. The apparent K m value for acetyl coenzyme A in the condensation reaction was 0.33 mM. The condensation reaction was inhibited by coenzyme A concentrations lower than 0.1 mM.
The enzyme was stable in the presence of dithiothreitol and other SH-compounds, but was strongly inhibited by 0.4 mM p-chloromercuribenzoate.
Similar content being viewed by others
Abbreviations
- PHB:
-
poly-β-hydroxybutyrate
References
Andrews, P.: Estimation of the molecular weights of proteins by Sephadex gel filtration. Biochem. J. 91, 222–233 (1964)
Beers, R. F., Jr., Sizer, I. W.: A spectrophotometric method for measuring the breakdown of hydrogen peroxide by catalase. J. Biol. Chem. 195, 133–140 (1952)
Bergmeyer, H. U., Gawehn, K., Klotzsch, H., Krebs, H. A., Williamson, D. H.: Purification and properties of crystalline 3-hydroxybutyrate dehydrogenase from Rhodopseudomonas spheroides. Biochem. J. 102, 423–431 (1967)
Berndt, H., Schlegel, H. G.: Kinetics and properties of β-ketothiolase from Clostridium pasteurianum. Arch. Microbiol. 103, 21–30 (1975)
Cleland, W. W.: Enzyme kinetics. In: Annu. Rev. Biochem., Vol. 36 (P. D. Boyer, A. Meister, R. L. Sinsheimer, E. E. Snell, eds.), pp. 77–112. Palo Alto: Annual Reviews 1967
Cleland, W. W.: Steady state kinetics. In: The enzymes, Vol. 2 (P. D. Boyer, ed.), pp. 1–65. New York-London: Academic Press 1970
Clinkenbeard, K. D., Sugiyama, T., Moss, J., Reed, W. D., Lane, M. D.: Molecular and catalytic properties of cytosolic acetoacetyl coenzyme A thiolase from avian liver. J. Biol. Chem. 248, 2275–2284 (1973)
Crabtree, K., McCoy, E., Boyle, W. C., Rohlich, G. A.: Isolation, identification, and metabolic role of the sudanophylic granules of Zoogloea ramigera. Appl. Microbiol. 13, 218–226 (1965)
Davis, B. J.: Disc electrophoresis. II. Method and application to human serum proteins. Ann. N.Y. Acad. Sci. 121, 404–427 (1964)
Flavin, M.: Methylmalonyl coenzyme A. In: Methods in enzymology, Vol. 6 (S. P. Colowick, N. O. Kaplan, eds.), pp. 538–539. New York-London: Academic Press 1963
Fukui, T., Yoshimoto, A., Matsumoto, M., Hosokawa, S., Saito, T., Nishikawa, H., Tomita, K.: Enzymatic synthesis of poly-β-hydroxybutyrate in Zoogloea ramigera. Arch. Microbiol. 110, 149–156 (1976)
Gehring, U., Riepertinger, C.: Dissoziation und Rekonstitution der Thiolase. European J. Biochem. 6, 281–292 (1968)
Gehring, U., Riepertinger, C., Lynen, F.: Reinigung und Kristallisation der Thiolase, Untersuchungen zum Wirkungsmechanismus. European J. Biochem. 6, 264–280 (1968)
Huth, W., Dierich, C., v. Oeynhausen, V., Seubert, W.: Multiple mitochondrial forms of acetoacetyl-CoA thiolase in rat liver: possible regulatory role in ketogenesis. Biochem. Biophys. Res. Commun. 56, 1069–1077 (1974)
Kornblatt, J. A., rudney, H.: Two forms of acetoacetyl coenzyme A thiolase in yeast. I. Separation and properties. J. Biol. Chem. 246, 4417–4423 (1971)
Layne, E.: Spectrophotometric and turbidimetric methods for measuring proteins. In: Methods in enzymology, Vol. 3 (S. P. Colowick, N. O. Kaplan, eds.), pp. 447–454. New York-London: Academic Press 1957
Martin, R. G., Ames, B. N.: A method for determining the sedimentation behavior of enzyme: Application to protein mixture. J. Biol. Chem. 236, 1372–1379 (1961)
Mazzei, Y., Negrel, R., Ailhaud, G.: Purification and some properties of thiolase from Escherichia coli. Biochim. Biophys. Acta 220, 129–131 (1970)
Middleton, B.: The kinetic mechanism and properties of the cytoplasmic acetoacetyl-coenzyme A thiolase from rat liver. Biochem. J. 139, 109–121 (1974)
Oeding, V., Schlegel, H. G.: β-Ketothiolase from Hydrogenomonas eutropha H 16 and its significance in the regulation of poly-β-hydroxybutyrate metabolism. Biochem. J. 134, 239–248 (1973)
Racker, E.: Alcohol dehydrogenase from baker's yeast. In Methods in enzymology, Vol. 1 (S. P. Colowick, N. O. Kaplan, eds.), pp. 500–503, New York-London: Academic Press 1955
Ritchie, G. A. F., Senior, P. J., Dawes, E. A.: The purification and characterization of acetoacetyl-coenzyme A reductase from Azotobacter beijerinckii. Biochem. J. 121, 309–316 (1971)
Saito, T., Fukui, T., Ikeda, F., Tanaka, Y., Tomita, K.: An NADP-linked acetoacetyl CoA reductase from Zoogloea ramigera. Arch. Microbiol. 114, 211–217 (1977)
Senior, P. J., Dawes, E. A.: The regulation of poly-β-hydroxybutyrate metabolism in Azotobacter beijerinckii. Biochem. J. 134, 225–238 (1973)
Simon, E., Shemin, D.: The preparation of S-succinyl coenzyme A. J. Am. Chem. Soc. 75, 2520 (1953)
Srere, P. A., Kosicki, G. W.: The purification of citrate-condensing enzyme. J. Biol. Chem. 236, 2557–2559 (1961)
Stern, J. R.: Crystalline crotonase from ox liver. In: Methods in enzymology, Vol. 1 (S. P. Colowick, N. O. Kaplan, eds.), pp. 559–566. New York-London: Academic Press 1955a
Stern, J. R.: Enzymes of acetoacetate formation and breakdown. In: Methods in enzymology, Vol. 1 (S. P. Colowick, N. O. Kaplan, eds.), pp. 573–585. New York-London: Academic Press 1955b
Tubbs, P. K., Garland, P. B.: Assay of coenzyme A and some acyl derivatives. In: Methods in enzymology, Vol. 13 (J. M. Lowenstein, ed.), pp. 535–551, New York-London: Academic Press 1969
Weber, K., Osborn, M.: The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J. Biol. Chem. 244, 4406–4412 (1969)
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Nishimura, T., Saito, T. & Tomita, K. Purification and properties of β-ketothiolase from Zoogloea ramigera . Arch. Microbiol. 116, 21–27 (1978). https://doi.org/10.1007/BF00408729
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF00408729