Abstract
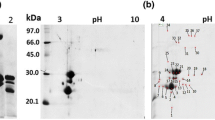
A glycoprotein (GP57) was purified by ion-exchange and hydroxylapatite column chromatography from the 70%-ethanol precipitate of culture medium of non-embryogenic carrot cells (Daucus carota L.) grown with 2,4-dichlorophen-oxyacetic acid (2,4-D). Its apparent molecular mass (M r) was estimated to be 57000 by sodium dodecylsulfate-polyacrylamide gel electrophoresis and 50000 by gel filtration. GP57 contained 14% (w/w) carbohydrate; the M r of the peptide portion was estimated to be 55000 after deglycosylation by trifluoromethanesulfonic acid. GP57 is composed of two polypeptides with the same Mr and with very similar amino-acid composition but different pI values, 8.8 and 9.5. Both are rich in aspartic acid, serine and threonine, and may possess N-linked oligosaccharide chains, including fucose and xylose. A monoclonal antibody (MAb) against the purified GP57 reacted with both the pI 8.8 and the 9.5 components, as well as the deglycosylated GP57. Immunoblotting with the MAb indicated that GP57 is synthesized in and released from cultured cells which have been supplied with auxin. In immunocytochemical studies, GP57 was found in the space between the embryo and the endosperm of dry seeds, and its content decreased during germination. GP57 was also found in the endodermis and epidermis of young roots, the periderm of mature taproots, and the epidermis of petioles and young leaves.
Similar content being viewed by others
Abbreviations
- 2,4-D:
-
2,4-dichlorophenoxyacetic acid
- GP57:
-
M r-57000 glycoprotein
- GP65:
-
M r-65000 glycoprotein
- MAb:
-
monoclonal antibody
- M r :
-
apparent molecular mass
- SDS-PAGE:
-
sodium dodecylsulfate-polyacrylamide gel electrophoresis
- TFMS:
-
trifluoromethanesulfonic acid
References
Albersheim, P., Nevins, D.J., English, P.D., Karr, A. (1967) A method for the analysis of sugars in plant cell-wall polysaccharides by gas-liquid chromatography. Carbohydr. Res. 5, 340–345
Bradford, M.M. (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72, 248–254
Dubois, M., Gilles, K.A., Hamilton, J.K., Rebers, P.A., Smith, F. (1956) Colorimetric method for determination of sugars and related substances. Anal. Chem. 28, 350–356
Edge, A.S.B., Faltynek, C.R., Hof, L., Reichert, L.E., Weber, P. (1981) Deglycosylation of glycoproteins by trifluoromethanesulfonic acid. Anal. Biochem. 118, 131–137
Esau, K. (1940) Developmental anatomy of the fleshy storage organ of Daucus carota. Hilgardia 13, 175–226
Esau, K., (1977) Anatomy of seed plants. John Wiley & Sons, New York, USA
Gefter, M.L., Margulies, D.H., Scharff, M.D. (1977) A simple method for polyethylene glycol-promoted hybridization of mouse myeloma cells. Somat. Cell Genet. 3, 231–236
Gershoni, J.M., Palade, G.E. (1982) Electrophoretic transfer of proteins from sodium dodecyl sulfate-polyacrylamide gels to a positively charged membrane filter. Anal. Biochem. 124, 396–405
Goding, J.W. (1980) Antibody production by hybridomas. J. Immunol. Meth. 39, 285–308
Ishihara, H., Takahashi, J., Oguri, S., Tejima, S. (1979) Complete structure of the carbohydrate moiety of Stem Bromelain. An application of the almond glycopeptidase for structural studies of glycopeptides. J. Biol. Chem. 254, 10715–10719
Kijimoto-Ochiai, S., Katagiri, Y.U., Ochiai, H. (1985) Analysis of N-linked oligosaccharide chains of glycoproteins on nitrocellulose sheets using lectin-peroxidase reagents. Anal. Biochem. 147, 222–229
Kolattukudy, P.E. (1984) Biochemistry and function of cutin and suberin. Can. J. Bot. 62, 2918–2933
Konat, G., Offner, H., Mellah, J. (1984) Improved sensitivity for detection and quantitation of glycoproteins on polyacrylamide gels. Experientia 40, 303–304
Laemmli, U.K. (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227, 680–685
Maruo, F., Okada, M. (1987) Monoclonal antibodies against Drosophila ovaries: their reaction with ovarian and embryonic antigens. Cell Different. 20, 45–54
Matsubara, H., Sasaki, R.M. (1969) High recovery of tryptophan from acid hydrolysates of protein. Biochem. Biophys. Res. Commun. 35, 175–181
McLean, I.W., Nakane, P.K. (1974) Periodate-lysine-paraformaldehyde fixative. A new fixative for immunoelectron microscopy. J. Histochem. Cytochem. 22, 1077–1083
Murashige, T., Skoog, F. (1962) A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol. Plant. 15, 473–497
Ouchterlony, O., Nilsson, L.A. (1973) Iminunodiffusion and immunoelectrophoresis. In: Handbook of Experimental Immunology vol. 1, pp. 19.1–19.39, (Weir, D.M., ed J.B. Lippincott Co., Philadelphia, Penn., USA
Piller, V., Piller, F., Cartron, J.-P. (1986) Isolation and characterization of an N-acetylgalactosamine specific lectin from Salvia sclarea seeds. J. Biol. Chem. 261, 14069–14075
Prigent, M.-J. (1984) Preliminary investigation of the structure of the carbohydrate component of Vicia graminea lectin, a plant glycoprotein. Carbohydr. Res. 131, 83–92
Satoh, S., Kamada, H., Harada, H., Fujii, T. (1986) Auxincontrolled glycoprotein release into the medium of embryogenic carrot cells. Plant Physiol. 81, 931–933
Satoh, S., Satoh, E., Watanabe, T., Fujii, T. (1985) Isolation and characterization of a protease inhibitor from spinach leaves. Phytochemistry 24, 419–423
Shibata, S., Goldstein, I.J., Baker, D.A. (1982) Isolation and characterization of a Lewis b-active lectin from Griffonia simplicifolia seeds. J. Biol. Chem. 257, 9324–9329
Steward, F.C., Mapes, M.O., Smith, J. (1958) Growth and organized development of cultured cells. I. Growth and division of freely suspended cells. Am. J. Bot. 45, 693–703
Streefkerk, J.G. (1972) Inhibition of erythrocyte pseudoperoxidase activity by treatment with hydrogen peroxide following methanol. J. Histochem. Cytochem. 20, 829–831
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Satoh, S., Fujii, T. Purification of GP57, and auxin-regulated extracellular glycoprotein of carrots, and its immunocytochemical localization in dermal tissues. Planta 175, 364–373 (1988). https://doi.org/10.1007/BF00396342
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00396342