Abstract
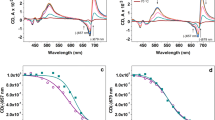
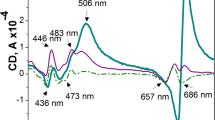
Changes in topography and function of pea (Pisum sativum L.) thylakoid membrane fractions following membrane protein phosphorylation have been studied. After protein phosphorylation the stromal membrane fraction had a higher chlorophyll a/b ratio, an increased content of light-harvesting chlorophyll protein and a higher ratio of chlorophyll to cytochrome f. This indicates that a pool of light-harvesting chlorophyll protein migrates from the photosystem II-enriched grana regions to the photosystem I-enriched stroma lamellae, in agreement with Kyle et al. (1984, Biochim. Biophys. Acta 765, 89–96) and Larsson et al. (1983, Eur. J. Biochem. 136, 25–29). Phosphorylation caused a stimulation in the rate of light-limited photosystem-I electron transfer in the unappressed membrane fraction, indicating that the translocated LHC-II becomes functionally associated with photosystem I.
Similar content being viewed by others
Abbreviations
- Fm:
-
fluorescence yield when all traps are closed
- Fo:
-
fluorescence yield when all traps are open
- Fv:
-
Fm-Fo
- LHC-II:
-
light-harvesting chlorophyll protein associated with photosystem II
- PSI, II:
-
photosystem I, II
- P700:
-
primary donor of PSI
References
Anderson, J.M., Melis, A. (1984) Localization of different photosystems in separate regions of chloroplast membranes. Proc. Natl. Acad. Sci. USA 80, 745–749
Anderson, J.M., Waldron, J.C., Thorne, S.W. (1978) Chlorophyll-protein complexes of spinach and barley thylakoids. FEBS. Lett. 92, 227–233
Andersson, B., Anderson, J.M. (1980) Lateral heterogeneity in the distribution of chlorophyll-protein complexes of the thylakoid membranes of spinach chloroplasts. Biochim. Biophys. Acta 539, 427–440
Barber, J. (1982) The control of membrane organisation by electrostatic forces. Biosci. Rep. 2, 1–13
Barber, J. (1983) Membrane conformational changes due to phosphorylation and the control of energy transfer in photosynthesis. Photochem. Photobiophys. 5, 181–190
Barber, J. (1985) Thylakoid membrane structure and organisation of electron transport components. In: Photosynthetic mechanisms and the environment, pp. 91–134, Barber, J., Baker, N.R., eds. Elsevier Biomedical Press, Amsterdam
Biggins, J. (1982) Thylakoid conformational changes accompanying membrane protein phosphorylation. Biochim. Biophys. Acta 679, 479–482
Black, M.T. (1985) Phosphorylation of thylakoid proteins and the distribution of excitation energy in pea chloroplasts. Ph.D. thesis, University of Sheffield, UK
Black, M.T., Foyer, C.H., Horton, P. (1984) An investigation into the ATP requirement for phosphorylation of thylakoid proteins and for the ATP-induced decrease in the yield of chlorophyll fluorescence in chloroplasts at different stages of development. Biochim. Biophys. Acta 767, 557–562
Black, M.T., Horton, P. (1984) An investigation into the mechanistic aspects of excitation energy redistribution following thylakoid membrane protein phosphorylation. Biochim. Biophys. Acta 767, 568–573
Bonaventura, C., Myers, J. (1969) Fluorescence and oxygen evolution from Chlorella pyrenoidosa. Biochim. Biophys. Acta 189, 363–383
Canaani, O., Malkin, S. (1984) Distribution of light excitation in an intact leaf between the two photosystems of photosynthesis. Changes in absorption cross-sections following state 1-state 2 transitions. Biochim. Biophys. Acta 766, 513–524
Cramer, W.A., Horton, P., Donnell, J.J. (1974) Inhibition of chemical oxidation and reduction of cytochromes f and b 559 by carbonylcyanide p-trifluoromethoxy phenylhydrazone. Biochim. Biophys. Acta 368, 361–370
Cramer, W.A., Whitmarsh, J. (1977) Photosynthetic cytochromes. Annu. Rev. Plant Physiol. 28, 133–172
Farchaus, J.W., Widger, W.R., Cramer, W.A., Dilley, R.A. (1982) Kinase induced changes in electron transport rates of spinach chloroplasts. Arch. Biochem. Biophys. 271, 362–367
Haworth, P., Kyle, D.J., Arntzen, C.J. (1982) Protein phosphorylation and excitation energy distribution in normal, ImL grown and a chlorophyll b-less mutant of barley. Arch. Biochem. Biophys. 218, 199–206
Haworth, P., Melis, A. (1983) Phosphorylation of chloroplast thylakoid membrane proteins does not increase the absorption cross-section of PSI. FEBS Lett. 160, 277–280
Haworth, P., Watson, J.L., Arntzen, C.J. (1983) The detection, isolation and characterisation of a light-harvesting complex which is specifically associated with PSI. Biochim. Biophys. Acta 724, 151–158
Horton, P. (1983) Control of chloroplast electron transport by phosphorylation of thylakoid proteins. FEBS Lett. 152, 47–52
Horton, P. (1985a) Regulation of photochemistry and its interaction with carbon metabolism. In: Regulation of sources and sinks in crop plants (Monograph 12) pp. 19–33. Jeffcoat, B., Hawkins, A.F., Stead, A.D., eds. British Plant Growth Regulator Group, Bristol
Horton, P. (1985b) Interactions between electron transfer and carbon assimilation. In: Photosynthetic mechanisms and the environment, pp. 135–187, Barber, J., Baker, N.R. eds. Elsevier Science Publishers B.V. (Biomedical Division), Amsterdam
Horton, P., Black, M.T. (1980) Activation of adenosine triphosphate-induced quenching of chlorophyll fluorescence by reduced plastoquinone. The basis of the state I-state II transition in chloroplast. FEBS Letters 119, 141–144
Horton, P., Black, M.T. (1981a) Light-dependent quenching of chlorophyll fluorescence in pea chloroplasts induced by ATP. Biochim. Biophys. Acta 635, 53–62
Horton, P., Black, M.T. (1981b) Light-induced redox changes in chloroplast cytochrome f after phosphorylation of membrane proteins. FEBS Lett. 132, 75–77
Horton, P., Black, M.T. (1982) On the nature of the fluorescence decrease due to phosphorylation of chloroplast membrane proteins. Biochim. Biophys. Acta 680, 22–27
Horton, P., Black, M.T. (1983) A comparison between cation and protein phosphorylation effects on the fluorescence induction curve in chloroplasts treated with 3-(3,4-dichlorophenyl)-1,1-dimethylurea. Biochim. Biophys. Acta 722, 214–218
Horton, P., Croze, E. (1977) The relationship between the activity of chloroplast photosystem II and the mid-point oxidation reduction potential of cytochrome b-559. Biochim. Biophys. Acta 462, 86–101
Krause, G.H., Behrend, U. (1983) Characterisation of chlorophyll fluorescence quenching in chloroplasts by fluorescence spectroscopy at 77K. II. ATP-dependent quenching. Biochim. Biophys. Acta 723, 176–181
Kyle, D.J., Haworth, P., Arntzen, C.J. (1982) Thylakoid membrane protein phosphorylation leads to a decrease in connectivity between PS2 reaction centres. Biochim. Biophys. Acta 680, 336–342
Kyle, D.J., Kuang, T.-Y., Watson, J.L., Arntzen, C.J. (1984) Movement of a sub-population of LHC-II from grana to stroma lamellae as a consequence of its phosphorylation. Biochim. Biophys. Acta 765, 89–96
Kyle, D.J., Staehelin, L.A., Arntzen, C.J. (1983) Lateral mobility of the light-harvesting chlorophyll protein in chloroplast membranes controls excitation energy distribution in higher plants. Arch. Biochem. Biophys. 222, 527–541
Larsson, U.K., Jergil, B., Andersson, B. (1983) Changes in the lateral distribution of the LHC a/b protein complex induced by its phosphorylation. Eur. J. Biochem. 136, 25–29
Markwell, J.P., Baker, N.R., Bradbury, M., Thorber, J.P. (1984) Use of zinc ions to study thylakoid protein phosphorylation and the state 1 to state 2 transition in vitro. Plant Physiol. 74, 348–354
Simpson, D.J. (1983) Freeze-fracture studies on barley plastid membranes. VII. Structural changes associated with phosphorylation of the LHC. Biochim. Biophys. Acta 725, 113–120
Staehelin, L.A., Arntzen, C.J. (1983) Regulation of chloroplast membrane function: Protein phosphorylation changes the spatial organisation of membrane components. J. Cell. Biol. 97, 1327–1337
Steinback, K.E., Bose, S., Kyle, D.J. (1982) Phosphorylation of the light-harvesting chlorophyll protein regulates excitation energy distribution between PS2 and PS1. Arch. Biochem. Biophys. 216, 356–361
Telfer, A., Hodges, M., Millner, P.A., Barber, J. (1984a) The cation-dependence of the degree of protein phosphorylation-induced unstacking of pea thylakoids. Biochim. Biophys. Acta 766, 554–562
Telfer, A., Bottin, H., Barber, J., Mathis, P. (1984b) The effect of magnesium and phosphorylation of the light-harvesting chlorophyll a/b protein on the yield of P700 photooxidation in pea chloroplasts. Biochim. Biophys. Acta 764, 324–330
Whitmarsh, J., Ort, D.R. (1984) Stoichiometries of electron transport complexes in spinach chloroplast. Arch. Biochem. Biophys. 231, 378–389
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Black, M.T., Lee, P. & Horton, P. Changes in topography and function of thylakoid membranes following membrane protein phosphorylation. Planta 168, 330–336 (1986). https://doi.org/10.1007/BF00392357
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00392357