Abstract
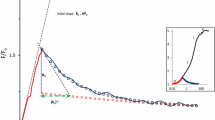
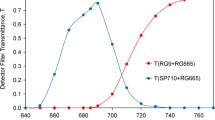
Light-induced fluorescence decay was examined during the greening of control and lincomycintreated maize (Zea mays L.) leaves. Assuming that this decay to a first approximation is the result of two parallel first-order reactions, the fluorescence induction curves were linearized on the logarithm plot and the parameters were determined. The variable fluorescence increased, and the parameters of the two linear sections of the fluorescence decay—that is, the kinetics of the induction curves—changed during the greening of the control leaves. Lincomycin treatment caused some chlorophyll deficiency and the lowering of the chlorophyll a/b ratio, changed the fluorescence emission spectra and the effect of Mg2+ on the regulation of the excitation energy distribution. The structure of the thylakoids and the kinetics of the fluorescence decay were also changed in the treated leaves. The possible relationship between the change of the kinetics of the fluorescence decay and the change of spillover during greening and after lincomycin treatment is discussed.
Similar content being viewed by others
Abbreviations
- LHC:
-
light-harvesting complex
- Chl:
-
chlorophyll
- LM:
-
lincomycin
- PS:
-
photosystem
- DCMU:
-
3-(3,4-dichlorophenyl)-1,1-dimethylurea
References
Armond, P.A., Arntzen, C.J., Briantais, J.-M., Vernotte, C.: Differentiation of chloroplast lamellae. Light harvesting efficiency and grana development. Arch. Biochem. Biophys. 175, 54–63 (1976)
Arntzen, C.J.: Dynamic structural features of chloroplast lamellae. In: Current topics in bioenergetics, Vol. VII. pp. 1–35, Sanadi, D.R., Vernon, L.P. eds. New York: Academic Press 1977
Boardman, N.K., Anderson, J.M., Hiller, R.G., Kahn, A., Roughan, P.G., Treffry, T.E., Thorne, S.W.: Biosynthesis of the photosynthetic apparatus during chloroplast development in higher plants. In: Proc. 2nd Int. Congress on Photosynthesis Research, Stresa, Vol. 3. pp. 2265–2287, Forti, G., Avron, M., Melandri, A., eds. The Hague, Dr. W. Junk. N.V. 1972
Davis, D.J., Armond, P.A., Gross, E.L., Arntzen, C.J.: Differentiation of chloroplast lamellae. Onset of cation regulation of excitation energy distribution. Arch. Biochem. Biophys. 175, 64–70 (1976)
Dubertret, G., Joliot, P.: Structure and organization of system II photosynthetic units during the greening of a dark-grown Chlorella mutant. Biochem. Biophys. Acta 357, 399–411 (1974)
Ellis, R.J.: Further similarities between chloroplast and bacterial ribosomes. Planta 91, 329–335 (1970)
Ellis, R.J.: Inhibition of chloroplast protein synthesis by lincomycin and 2-(4-methyl-2,6-dinitroanilino)-N-methyl propionamid. Phytochemistry 14, 89–93 (1975)
Forger, J.M., Bogorad, L.: Steps in aquisition of photosynthetic competence by plastids of maize. Plant Physiol. 52, 491–497 (1973)
Hiller, R.G., Pilger, T.B.G., Genge, S.: Effect of lincomycin on the chlorophyll-protein complex I content and photosystem I activity of greening leaves. Biochim. Biophys. Acta 460, 431–444 (1977)
Horváth, G., Garab, Gy.I., Mustárdy, L.A., Halász, N., Faludi-Dániel, Á.: The development of thylakoids and photochemical properties of mesophyll and bundle sheath chloroplasts of greening maize leaves. Plant Sci. Lett. 5, 239–244 (1975)
Ichikawa, T., Inoue, Y., Shibata, K.: Delayed light emission and variable fluorescence from intermittently illuminated wheat leaves under continuous illumination related to activation of the latent water-splitting system. Plant Sci. Lett. 4, 369–376 (1975)
Klimov, V.V., Láng, F., Karapetyan, N.V., Krasnovsky, A.A.: Fluorescence induction during the greening of etiolated leaves: normal and mutant maize seedlings. Fiziol. Rast. 19, 151–159 (1972)
Krause, G.H.: Changes in chlorophyll fluorescence in relation to light-dependent cation transfer across thylakoid membranes. biochem. Biophys. Acta 333, 301–313 (1974)
Mills, J., Barber, J.: Energy-dependent cation-induced control of chlorophyll a fluorescence in isolated intact chloroplasts. Arch. Biochem. Biophys. 170, 306–314 (1975)
Mohanty, P., Govindjee: Light induced changes in the fluorescence yield of chlorophyll a in Ancystis nidulans. I. Relationship of slow fluorescence changes with structural changes. Biochim. Biophys. Acta 305, 95–104 (1973)
Murata, N.: Control of excitation energy transfer in photosynthesis. V. Correlation membrane structure to regulation of excitation transfer between two pigment systems in isolated chloroplasts. Biochim. Biophys. Acta 245, 365–372 (1971)
Murata, N., Tashiro, H., Takamiya, A.: Effects of divalent metal ions on chlorophyll a fluorescence in isolated spinach chloroplasts. Biochim. Biophys. Acta 197, 250–256 (1970)
Papageorgiou, G.: Chlorophyll fluorescence: An intrinsic probe of photosynthesis. In: Bioenergetics of photosynthesis, pp. 319–371. Govindjee, ed. New York-San Francisco-London: Academic Press 1975
Papageorgiou, G., Issakidou, J., Argoudelis, C.: Structure dependent control of chlorophyll a excitation density: the role of oxygen. FEBS Lett. 25, 139–142 (1972)
Sárvári, É., Halász, G., Nytrai, P., Láng, F.: Effect of lincocin treatment on the greening process in bean (Phaseolus vulgaris) leaves. Physiol. Plant. 36, 187–192 (1976)
Schreiber, U., Fink, R., Vidaver, W.: Fluorescence induction in whoole leaves: Differentiation between the two sides and adaptation to different light regimes. Planta 133, 121–129 (1977)
Sokolove, P.M., Marsho, T.V.: Slow fluorescence quenching of type A chloroplasts resolution into two components. Biochim. Biophys. Acta 459, 27–35 (1977)
Strasser, R.J., Sironval, C.: Correlation between the induction of oxygen evolution and of variable fluorescence in flashed bean leaves. Plant Sci. Lett. 3, 135–141 (1974)
Telfer, A., Barber, J., Nicolson, J.: Energy-dependent quenching of chlorophyll a fluorescence. Evidence for coupled cyclic electron flow in isolated intact chloroplasts. Plant Sci. Lett. 5, 171–176 (1975)
Thomson, W.W., Ellis, R.J.: Inhibition of grana formation by lincomycin. Planta 108, 89–92 (1972)
Vernotte, C., Briantais, J.-M., Artzen, C.J.: Comparison of excitons transfers changes induced by cations and by adaptation in states I and II. In: Proc. 3rd. Int. Congress on Photosynthesis, Rehovot, pp. 183–193 Avron, M. ed.. Amsterdam: Elsevier 1974
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Sárvári, É., Halász, G., Török, S. et al. Light-induced fluorescence decay during the greening of normal and lincomycin-treated maize leaves. Planta 141, 135–139 (1978). https://doi.org/10.1007/BF00387879
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00387879