Abstract
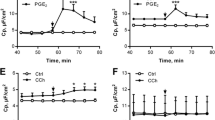
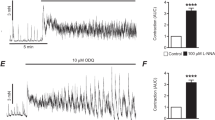
Whole-cell recordings were performed at isolated crypts from the distal colon of the rat. Enterocytes in intact crypts, patched from the basolateral side, exhibited a gradient in the resting zero-current potential. Along the axis of the crypt, the highest potentials were measured in the ground region, the lowest in the surface region. The cholinergic agonist, carbachol, induced a hyperpolarization and an increase of the outward current in both the middle and the ground cells of intact crypts. This effect could be prevented by Ba2+ or by the intracellular Ca2+ antagonist, 8-(N, N-diethylamino)-octyl-3,4,5-trimethoxy-benzoate hydrochloride (TMB-8). Its action, however, was not dependent on the presence of external Ca2+. Both ground cells and the cells in the middle part of the crypt responded to forskolin, an activator of the adenylate cyclase, with a depolarization. In the middle part of the crypt, the depolarization induced by forskolin was associated with an increase of the outward current. It could be blocked by the Cl− channel blocker, 5-nitro-2-(3-phenylpropylamino)-benzoate, indicating an increase of Cl− conductance. In contrast, the forskolin-induced depolarization in the ground part of the crypt was associated with a decrease of the outward current. This effect could be prevented by Ba2+, indicating a decrease of a potassium conductance. The changes in outward current could be prevented by the presence of an inhibitor of protein kinase A in the pipette solution. In conclusion, these results suggest that carbachol, an agonist acting on the Ca2+ pathway, indirectly causes Cl− secretion by an increase of the driving force, i.e. the membrane potential. Only the activation of cyclic AMP synthesis by forskolin is able to increase Cl− conductance in the rat colon. The latter response seems to be dependent on the state of differentiation of the enterocytes.
Similar content being viewed by others
References
Abdel-Latif AA (1986) Calcium-mobilizing receptors, polyphosphoinositides, and the generation of second messengers. Pharmacol Rev 3:227–272
Andres H, Bock R, Bridges RJ, Rummel W, Schreiner J (1985) Submucosal plexus and electrolyte transport across rat colonic mucosa. J Physiol (Lond) 364:301–312
Ashby CD, Walsh DA (1972) Characterization of a protein inhibitor with adenosine 3′,5′-monophosphate-dependent protein kinases. J Biol Chem 247:6637–6642
Binder HJ, Sandle GI (1987) Electrolyte absorption and secretion in the mammalian colon. In: Johnson LR (ed) Physiology of the gastrointestinal tract. Raven, New York, pp 1389–1418
Bjerknes M, Cheng H, Erlandsen S (1985) Functional gap junctions in mouse small intestinal crypts. Anat Rec 212:364–367
Bolton JE, Field M (1977) Ca ionophore-stimulated ion secretion in rabbit ileal mucosa: Relation to actions of cyclic 3′,5′-AMP and carbamylcholine. J Membr Biol 35:159–177
Bridges RJ, Rummel W, Simon B (1983) Forskolin induced chloride secretion across the isolated mucosa of rat colon descendens. Naunyn-Schmiedeberg's Arch Pharmacol 323:355–360
Cartwright CA, McRoberts JA, Mandel KG, Dharmsathaphorn K (1985) Synergistic action of cyclic adenosine monophosphate- and calcium-mediated chloride secretion in a colonic epithelial cell line. J Clin Invest 76:1837–1842
Cohn JA (1990) Protein kinase C mediates cholinergically regulated protein phosphorylation in a Cl− secreting epithelium. Am J Physiol 258:C227-C233
Corey DP, Stevens CF (1983) Science and technology of patch-recording electrodes. In: Sakmann B, Neher E (eds) Single-channel recording. Plenum P, New York London, pp 53–68
Cremaschi D, James PS, Meyer G, Peacock MA, Smith WM (1982) Membrane potentials of differentiating enterocytes. Biochim Biophys Acta 688:271–274
Curtis RL, Trier JS, Frizzell RA, Lindem NM, Madara JL (1984) Flounder intestinal absorptive cells have abundant gap junctions and may be coupled. Am J Physiol 246:C77-C83
Cuthbert AW, Spayne JA (1982) Stimulation of sodium and of chloride transport in epithelia by forskolin. Br J Pharmacol 76:33–35
Del Castillo JR (1987) The use of hyperosmolar, intracellular-like solutions for the isolation of epithelial cells from guinea-pig small intestine. Biochim Biophys Acta 901:201–208
Devor DC, Simasko SM, Duffey ME (1990) Carbachol induces oscillations of membrane potassium conductance in a colonic cell line, T84. Am J Physiol 258:C318-C326
Dharmsathaphorn K, Pandol SJ (1986) Mechanism of chloride secretion induced by carbachol in a colonic epithelial cell line. J Clin Invest 77:348–354
Diener M (1987) Patch clamp data on isolated enterocytes from rat colon descendens: Ca2+ dependent regulation of K+ conductance. Z Gastroenterol 25:624
Diener M, Rummel W (1989) Actions of the chloride channel blocker NPPB on absorptive and secretory transport processes in rat colon. Acta Physiol Scand 137:215–222
Diener M, Eglème C, Rummel W (1991) Phospholipase C-induced anion secretion and its interaction with carbachol in the rat colonic mucosa. Eur J Pharmacol (in press)
Diener M, Rummel W, Mestres P, Lindemann B (1989) Single chloride channels in colon mucosa and isolated colonic enterocytes of the rat. J Membr Biol 108:21–30
Donowitz M, Fogel F, Battisti L, Asarkof N (1982) The neurohumoral secretagogues carbachol, substance P and neurotensin increase Ca2+ influx and calcium content in rabbit ileum. Life Sci 31:1929–1937
Frizzell RA, Halm DR, Rechkemmer G, Shoemaker RL (1986) Chloride channel regulation in secretory epithelia. Fed Proc 45:2727–2731
Frizzell RA, Rechkemmer GR, Shoemaker RL (1986) Altered regulation of airway epithelial cell chloride channels in cystic fibrosis. Science 233:558–560
Giraldez F, Sepúlveda FV, Sheppard DN (1988) A chloride conductance activated by adenosine 3′,5′-cyclic monophosphate in the apical membrane of Necturus enterocytes. J Physiol (Lond) 395:597–623
Giraldez F, Murray KJ, Sepúlveda FV, Sheppard DN (1989) Characterization of a phosphorylation-activated Cl–-selective channel in isolated Necturus enterocytes. J Physiol (Lond) 415:517–537
Gögelein H, Pfannmüller B (1989) The nonselective cation channel in the basolateral membrane of rat exocrine pancreas. Inhibition by 3′,5′-dichlorophenylamine-2-carboxylic acid (DCDPC) and activation by stilbene disulfonates. Pflügers Arch 413:287–298
Greger R, Schlatter E, Gögelein H (1985) Cl–-channels in the apical membrane of the rectal gland “induced” by cAMP. Pflügers Arch 403:446–448
Halm DR, Rechkemmer G, Schoumacher RA, Frizzel RA (1988) Apical membrane chloride channels in a colonic cell line activated by secretory agonists. Am J Physiol 254:C505-C511
Hamill OP, Marty A, Neher E, Sakmann B, Sigworth FJ (1981) Improved patch-clamp technique for high-resolution current recordings from cells and cell-free membrane patches. Pflügers Arch 391:85–100
Hardcastle J, Hardcastle PT, Noble JM (1984) The involvement of calcium in the intestinal response to secretagogues in the rat. J Physiol (Lond) 355:465–478
Heuschneider G, Schwartz RD (1989) cAMP and forskolin decrease gamma-aminobutyric acid-gated chloride flux in rat brain synaptosomes. Proc Natl Acad Sci USA 86:2938–2942
Horn R, Marty A (1988) Muscarinic activation of ionic currents measured by a new whole-cell recording method. J Gen Physiol 92:145–159
Hoshi T, Garber SS, Aldrich RW (1988) Effect of forskolin on voltage-gated K+ channels is independent of adenylate cyclase activation. Science 240:1652–1655
Kataoka K, Tabata J, Yamamoto M, Toyota T (1989) The association of gap junctions with large particles in the crypt epithelium of the rat small intestine. Arch Histol Cytol 52:81–86
Lipkin M (1987) Proliferation and differentiation of normal and diseased gastrointestinal cells. In Johnson LR (ed), Physiology of the gastrointestinal tract, Raven, New York, pp 255–284
Loewenstein WR, Kanno Y (1964) Studies on epithelial (gland) cell junction: I. Modifications of surface membrane permeability. J Cell Biol 22:565–586
Loo DDF, Kaunitz JD (1989) Ca2+ and cAMP activate K+ channels in the basolateral membrane of crypt cells isolated from rabbit distal colon. J Membr Biol 110:19–28
Malagodi MH, Chiou CY (1974) Pharmacological evaluation of a new Ca2+ antagonist, 8-(N,N-diethylamino)-octyl-3,4,5-trimethoxybenzoate hydrochloride (TMB-8): studies in smooth muscle. Eur J Pharmacol 27:25–33
Morris AP, Gallacher DV, Lee JAC (1986) A large conductance, voltageand calcium-activated K+ channel in the basolateral membrane of rat enterocytes. FEBS Lett 206:87–92
Morris AP, Kirk KL, Frizzell RA (1990) Simultaneous analysis of cell Ca2+ and Ca2+-stimulated chloride conductance in colonic epithelial cells (HT29). Cell Regul 1:951–963
Rimele TJ, O'Dorisio MS, Gaginella TS (1981) Evidence for muscarinic receptors on rat colonic epithelial cells. Binding of [3H]quinuclidinyl benzilate. J Pharmacol Exp Ther 218:426–434
Seamon KB, Padgett W, Daly JW (1981) Forskolin: unique diterpene activator of adenylate cyclase in membranes and intact cells. Proc Natl Acad Sci USA 78:3363–3367
Sepúlveda FV, Mason WT (1985) Single channel recordings obtained from basolateral membranes of isolated rabbit enterocytes. FEBS Lett 191:87–91
Sheppard DN, Giraldez F, Sepúlveda FV (1988) Kinetics of voltage and Ca2+ activation and Ba2+ blockade of a large-conductance K+ channel from Necturus small intestine. J Membr Biol 105:65–75
Spray DC, Bennett MVL (1985) Physiology and pharmacology of gap junctions. Annu Rev Physiol 47:281–303
Stewart CP, Turnberg LA (1989) A microelectrode study of responses to secretagogues by epithelial cells on villus and crypt of rat small intestine. Am J Physiol 257:G334-G343
Wangemann P, Wittner M, Di Stefano A, Englert HC, Lang HJ, Schlatter E, Greger R (1986) Cl−-channel blockers in the thick ascending limb of the loop of Henle. Structure activity relationship. Pflügers Arch 407:S128–141
Welsh MJ, Smith PL, Fromm M, Frizzel RA (1982) Crypts are the site of intestinal fluid and electrolyte secretion. Science 218:1219–1221
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Böhme, M., Diener, M. & Rummel, W. Calcium- and cyclic-AMP-mediated secretory responses in isolated colonic crypts. Pflügers Arch. 419, 144–151 (1991). https://doi.org/10.1007/BF00373000
Received:
Revised:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00373000