Summary
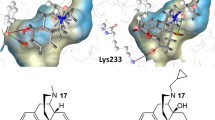
Cyclic β-casomorphin analogues with a d-configured amino acid residue in position 2, such as Tyr-c[-Xaa-Phe-Pro-Gly-] and Tyr-c[-Xaa-Phe-d-Pro-Gly-] (Xaa=d-A2bu, d-Orn, d-Lys) were found to bind to the μ-opioid receptor as well as to the δ-opioid receptor, whereas the corresponding l-Xaa2 derivatives are nearly inactive at both. Low-energy conformers of both active and nearly inactive derivatives have been determined in a systematic conformational search or by molecular dynamics simulations using the TRIPOS force field. The obatained conformations were compared with regard to a model for μ-selective opiates developed by Brandt et al. [Drug Des. Discov., 10 (1993) 257]. Superpositions as well as electrostatic, lipophilic and hydrogen bonding similarities with the δ-opioid receptor pharmacophore conformation of t-Hpp-JOM-13 proposed by Mosberg et al. [J. Med. Chem., 37 (1994) 4371, 4384] were used to establish the probable δ-pharmacophoric cyclic β-casomorphin conformations. These conformations were also compared with a δ-opioid agonist (SNC 80) and the highly potent antagonist naltrindole. These investigations led to a prediction of the μ-and δ-pharmacophore structures for the cyclic β-casomorphins. Interestingly, for the inactive compounds such conformations could not be detected. The comparison between the μ-and δ-pharmacophore conformations of the cyclic β-casomorphins demonstrates not only differences in spatial orientation of both aromatic groups, but also in the backbone conformations of the ring part. In particular, the differences in Φ2 and Ψ2 (μ≈70°,-80°; δ≈165°,55°) cause a completely different spatial arrangement of the cyclized peptide rings when all compounds are matched with regard to maximal spatial overlap of the tyrosine residue. Assuming that both the μ-and δ-pharmacophore conformations bind with the tyrosine residue in a similar orientation at the same transmembrane domain X of their receptors, the side chain of Phe3 as a second binding site has to dock with different domains.
Similar content being viewed by others
References
Brantl, V., Teschemacher, H., Henschen, A. and Lottspeich, F., Hoppe-Seyler's Z. Physiol. Chem., 360 (1979) 1211.
Henschen, A., Lottspeich, F., Brantl, V. and Teschemacher, H., Hoppe-Seyler's Z. Physiol. Chem., 360 (1979) 1217.
Koch, G. and Brantl, V., In Nyberg, F. and Brantl, V. (Eds.) β-Casomorphins and Related Peptides, Fyris-Tryck AB, Uppsala, Sweden, 1990, pp. 43–52.
Brantl, V., Pfeiffer, A., Herz, A., Henschen, A. and Lottspeich, F., Peptides, 3 (1982) 793.
Liebmann, C., Szücs, M., Neubert, K., Hartrodt, B., Arold, H. and Barth, A., Peptides, 7 (1986) 195.
Chang, K.J., Wie, E.T., Killian, A. and Chang, J.K., J. Pharmacol. Exp. Ther., 227 (1983) 403.
Schmidt, R., Neubert, K., Barth, A., Liebmann, C., Schnittler, M., Chung, N.N. and Schiller, P.W., Peptides, 12 (1991) 1175.
Rüthrich, H.-L., Grecksch, G., Schmidt, R. and Neubert, K., Peptides, 13 (1992) 483.
Schmidt, R., Vogel, D., Mrestani-Klaus, C., Brandt, W., Neubert, K., Chung, N.N., Lemieux, C. and Schiller, P.W., J. Med. Chem., 37 (1994) 1136.
Brandt, W., Mrestani-Klaus, C., Schmidt, R., Schinke, H., Neubert, K., Schiller, P.W., Höltje, H.-D. and Barth, A., Quant. Struct.-Act. Relatsh., 14 (1995) 417.
Kleinpeter, E., Ströhl, D., Peinze, S., Brandt, W., Schmidt, R. and Neubert, K., Struct. Chem., 7 (1996) 139–151, in press.
Mrestani-Klaus, C., Brandt, W., Schmidt, R., Schiller, P.W. and Neubert, K., Pharm. Med. Chem, 329 (1996) 133.
Keys, C., Payne, P., Amsterdam, P., Toll, L. and Loew, G., Mol. Pharmacol., 33 (1988) 528.
Froimowitz, M. and Hruby, V.J., Int. J. Pept. Protein Res., 34 (1989) 88.
Nikiforovich, G.V. and Hruby, V.J., Biochem. Biophys. Res. Commun., 173 (1990) 521.
Nikiforovich, G.V., Golbraikh, A., Shenderovich, M.D. and Balodis, J., Int. J. Pept. Protein Res., 36 (1990) 209.
Chew, C., Villar, H.O. and Loew, G.H., Biopolymers, 33 (1993) 647.
Mosberg, H.I., Lomize, A.L., Wang, C., Kroona, H., Heyl, D.L., Sobczyk-Kojiro, K., Ma, W., Mousigian, C. and Porreca, F., J. Med. Chem., 37 (1994) 4371.
Mosberg, H.I., Omnaas, J.R., Lomize, A.L., Wang, C., Heyl, D.L., Nordan, I., Mousigian, C., Davis, P. and Porreca, F., J. Med. Chem., 37 (1994) 4384.
Calderon, S.N., Rothman, R.B., Porreca, F., Flippen-Anderson, J.L., McNutt, R.W., Xu, H., Smith, L.E., Bilsky, E.J., Davis, P. and Rice, K.C., J. Med. Chem., 37 (1994) 2125.
Portoghese, P.S., Sultana, M., Moe, S.T. and Takemori, A.E., J. Med. Chem., 37 (1994) 579.
Brandt, W., Barth, A. and Höltje, H.-D., Drug Des. Discov., 10 (1993) 257.
Penkler, L.J., VanRooyen, P.H. and Wessels, P.L., Int. J. Pept. Protein Res., 41 (1993) 261.
Maigret, B., Fournie-Zaluski, M.C., Roques, B. and Premilat, S., Mol. Pharmacol., 29 (1986) 314.
Clark, M., Cramer III, R.D. and VanOpdenbosch, N., J. Comput. Chem., 10 (1989) 982.
SYBYL, Tripos Associates Inc., St. Louis, MO.
Gasteiger, J. and Marseli, M., Tetrahedron, 36 (1980) 3219.
Gasteiger, J. and Saller, H., Angew. Chem., 97 (1985) 699.
Brandt, J., Wahab, M., Thondorf, I., Schinke, H. and Barth, A., J. Mol. Graph., 9 (1991) 122.
Cometta-Morini, C. and Loew, G.H., J. Comput.-Aided Mol. Design, 5 (1991) 335.
Liebmann, C., Schnittler, M., Hartrodt, B., Born, I. and Neubert, K., Pharmazie, 46 (1991) 345.
Neubert, K., Hartrodt, B., Born, I., Barth, A., Ruethrich, H.-L., Grecksch, G., Schrader, U. and Liebmannin, C., In Nyberg, F. and Brantl, V. (Eds.) β-Casomorphins and Related Peptides, Fyris-Tryck AB, Uppsala, Sweden, 1990, pp. 15–20.
Schmidt, R., Kalman, A., Chung, N.N., Lemieux, C., Horvath, C. and Schiller, P.W., Int. J. Pept. Protein Res., 46 (1995) 47.
Schiller, P.W., Nguyen, T.M.-D. and Lemieux, C., Adv. Biosci., 75 (1989) 85.
Schwyzer, R., Biochemistry, 25 (1986) 6335.
Melchiorri, P., Negri, L., Falconieri-Espamer, G., Severini, C., Corsi, R., Soaje, M., Erspamer, V. and Barra, D., Eur. J. Pharmacol., 195 (1991) 201.
Lazarus, L.H., Salvadori, S., Santagada, V., Tomatis, R. and Wilson, W.E., J. Med. Chem., 34 (1991) 1350.
Portoghese, P.S., Moe, S.T. and Takemori, A.E., J. Med. Chem., 36 (1993) 2572.
Kong, H., Raynor, K., Yasuda, K., Moe, S.T., Portoghese, P.S., Bell, G.I. and Reisine, T., J. Biol. Chem., 268 (1993) 23055.
Author information
Authors and Affiliations
Additional information
This paper is based on a presentation given at the 14th Molecular Graphics and Modelling Society Conference, held in Cairns, Australia, August 27–September 1, 1995.
Rights and permissions
About this article
Cite this article
Brandt, W., Stoldt, M. & Schinke, H. The μ-and δ-opioid pharmacophore conformations of cyclic β-casomorphin analogues indicate docking of the Phe3 residue to different domains of the opioid receptors. Journal of Computer-Aided Molecular Design 10, 201–212 (1996). https://doi.org/10.1007/BF00355043
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00355043