Summary
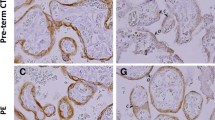
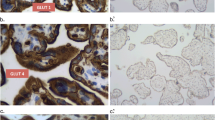
The syncytiotrophoblast covering the surface of the placental villi contains the machinery for the transfer of specific substances between maternal and fetal blood, and also serves as a barrier. Existence of a facilitated-diffusion transporter for glucose in the syncytiotrophoblast has been suggested. Using antibodies to erythrocyte/HepG2-type glucose transporter (GLUT1), one isoform of the facilitated-diffusion glucose transporters, we detected a 50 kD protein in human placenta at term. By use of immunohistochemistry, GLUT1 was found to be abundant in both the syncytiotrophoblast and cytotrophoblast. Endothelial cells of the fetal capillaries also showed positive staining for GLUT1. Electron-microscopic examination revealed that GLUT1 was concentrated at both the microvillous apical plasma membrane and the infolded basal plasma membrane of the syncytiotrophoblast. Plasma membrane of the cytotrophoblast was also positive for GLUT1. GLUT1 at the apical plasma membrane of the syncytiotrophoblast may function for the entry of glucose into its cytoplasm, while GLUT1 at the basal plasma membrane may be essential for the exit of glucose from the cytoplasm into the stroma of the placental villi. Thus, GLUT1 at the plasma membranes of syncytiotrophoblast and endothelial cells may play an important role in the transport of glucose across the placental barrier.
Similar content being viewed by others
References
Baly DL, Horuk R (1988) The biology and biochemistry of the glucose transporter. Biochim Biophys Acta 947:571–590
Bell GI, Kayano T, Buse JB, Burant CF, Takeda J, Lin D, Fukumoto H, Seino S (1990) Molecular biology of mammalian glucose transporters. Diabetes Care 13:198–208
Benirschke K, Kaufmann P (1990) Basic structure of the villous trees. In: Pathology of the human placenta, 2nd edn. Springer, New York, pp 22–70
Bissonnette JM, Black JA, Wickham WK, Acott KM (1981) Glucose uptake into plasma membrane vesicles from the maternal surface of human placenta. J Membr Biol 58:75–80
Bissonnette JM, Black JA, Thornburg KL, Acott KM, Koch PL (1982) Reconstitution of D-glucose transporter from human placental microvillous plasma membranes. Am J Physiol 242:C166–C171
Challier JC, Hauguel S, Desmaizieres V (1986) Effect of insulin on glucose uptake and metabolism in the human placenta. J Clin Endocrinol Metab 62:803–807
Coady MJ, Pajor M, Wright EM (1990) Sequence homologies among intestinal and renal Na+/glucose cotransporters. Am J Physiol 259:C605–C610
Dancis J, Schneider H (1975) Physiology: transfer and barrier function. In: Gruenwald P (ed) The placenta and its maternal supply line. Medical and Technical Publishing Co, Lancaster, pp 98–124
DeMey J (1984) Colloidal gold as marker and tracer in light and electron microscopy. EMSA Bulletin 14:54–66
Ezaki O (1990) The insulin-like effects of selenate in rat adipocytes. J Biol Chem 265:1124–1128
Ezaki O, Kasuga M, Akanuma Y, Takata K, Hirano H, Fujita-Yamaguchi Y, Kasahara M (1986) Recycling of the glucose transporter, the insulin receptor, and insulin in rat adipocytes. Effect of acidtropic agents. J Biol Chem 261:3295–3305
Fukumoto H, Seino S, Imura H, Seino Y, Bell GI (1988) Characterization and expression of human HepG2/erythrocyte glucose-transporter gene. Diabetes 37:657–661
Gould GW, Bell GI (1990) Facilitative glucose transporters: an expanding family. Trends Biochem Sci 15:18–23
Harik SI, Kalaria RN, Whitney PM, Andersson L, Lundahl P, Ledbetter SR, Perry G (1990a) Glucose transporters are abundant in cells with “occluding” junctions at the blood-eye barriers. Proc Natl Acad Sci USA 87:4261–4264
Harik SI, Kalaria RN, Andersson L, Lundahl P, Perry G (1990b) Immunocytochemical localization of the erythroid glucose transporter: Abundance in tissues with barrier functions. J Neurosci 10:3862–3872
Hediger MA, Coady MJ, Ikeda TS, Wright EM (1987) Expression cloning and cDNA sequencing of the Na+/glucose co-transporter. Nature 330:379–381
Heinrich D, Metz J, Raviola E, Forssmann WG (1976) Ultrastructure of perfusion-fixed fetal capillaries in the human placenta. Cell Tissue Res 172:157–169
Ingermann RL, Bissonnette JM, Koch PL (1983) D-glucose-sensitive and-insensitive cytochalasin B binding proteins from microvillous plasma membranes of human placenta. Identification of the D-glucose transporter. Biochim Biophys Acta 730:57–63
James DE, Strube M, Mueckler M (1989) Molecular cloning and characterization of an insulin-regulatable glucose transporter. Nature 338:83–87
Johnson LW, Smith CH (1980) Monosaccharide transport across microvillous membrane of human placenta. Am J Physiol 238:C160–168
Johnson LW, Smith CH (1982) Identification of the glucose transport protein of the microvillous membrane of human placenta by photoaffinity labelling. Biochem Biophys Res Commun 30:408–413
Johnson LW, Smith CH (1985) Glucose transport across the basal plasma membrane of human placental syncytiotrophoblast. Biochim Biophys Acta 815:44–50
Kasahara M, Hinkle PC (1977) Reconstitution and purification of the D-glucose transporter from human erythrocytes. J Biol Chem 252:7384–7390
Kasahara M, Inui K, Takano M, Hori R (1985) Distinction of three types of D-glucose transport systems in animal cells. Biochem Biophys Res Commun 132:490–496
Kelley LK, Smith CH, King BF (1983) Isolation and partical characterization of the basal cell membrane of human placental trophoblast. Biochim Biophys Acta 734:91–98
Morriss FH, Boyd RDH (1988) Placental transport. In: Knobil E, Neill JD (eds) The physiology of reproduction. Raven Press, New York, pp 2043–2083
Mueckler M, Caruso C, Baldwin SA, Panico M, Blench I, Morris HR, Allard WJ, Lienhard GE, Lodish HF (1985) Sequence and structure of a human glucose transporter. Science 229:941–945
Sase S, Takata K, Hirano H, Kasahara M (1982) Characterization and identification of the glucose transporter of human erythrocytes. Biochim Biophys Acta 693:253–261
Siegel TW, Ganguly S, Jacobs S, Rosen OM, Rubin CS (1981) Purification and properties of the human placental insulin receptor. J Biol Chem 256:9266–9273
Slot JW, Geuze HJ (1985) A new method of preparing gold probes for multiple-labeling cytochemistry. Eur J Cell Biol 38:87–93
Takata K, Singer SJ (1988) Phosphotyrosine-modified proteins are concentrated at the membranes of epithelial and endothelial cells during tissue development in chick embryos. J Cell Biol 106:1757–1764
Takata K, Hirano H (1990) Use of fluorescein-phalloidin and DAPI as a counterstain for immunofluorescence microscopic studies with semithin frozen sections. Acta Histochem Cytochem 23:679–683
Takata K, Kasahara T, Kasahara M, Ezaki O, Hirano H (1990) Erythrocyte/HepG2-type glucose transporter is concentrated in cells of blood-tissue barriers. Biochem Biophys Res Commun 173:67–73
Takata K, Kasahara T, Kasahara M, Ezaki O, Hirano H (1991a) Localization of Na+-dependent active type and erythrocyte/HepG2-type glucose transporters in rat kidney: Immunofluorescence and immunogold study. J Histochem Cytochem 39:287–298
Takata K, Kasahara T, Kasahara M, Ezaki O, Hirano H (1991b) Ultracytochemical localization of erythrocyte/HepG2-type glucose transporter (GLUT1) in the ciliary body and iris of the rat eye. Invest Ophthalmol Vis Sci 32:1659–1666
Thorens B, Charron MJ, Lodish HF (1990a) Molecular physiology of glucose transporters. Diabetes Care 13:209–218
Thorens B, Lodish HF, Brown D (1990b) Differential localization of two glucose transporter isoforms in rat kidney. Am J Physiol 259:C286–C294
Tokuyasu KT (1986) Application of cryoultramicrotomy to immunocytochemistry. J Microsc 143:139–149
Wessling M, Pilch PF (1984) Characterization and solubilization of the cytochalasin B binding component from human placental microsomes. Biochim Biophys Acta 777:123–132
Wheeler TJ, Hinkle PC (1985) The glucose transporter of mammalian cells. Ann Rev Physiol 47:503–517
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Takata, K., Kasahara, T., Kasahara, M. et al. Localization of erythrocyte/HepG2-type glucose transporter (GLUT1) in human placental villi. Cell Tissue Res 267, 407–412 (1992). https://doi.org/10.1007/BF00319362
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00319362