Summary
A study was carried out in 11 healthy young men to investigate the pharmacokinetics of chlorpromazine (CPZ) after a bolus intravenous (IV) dose (10 mg) and three single oral doses (25, 50 and 100 mg), with a washout period of two weeks between doses. Plasma levels of CPZ, CPZ N-oxide (CPZNO), CPZ sulfoxide (CPZSO) and both free and conjugated 7-hydroxy-CPZ (7-HOCPZ) were measured by extraction radioimmunoassays.
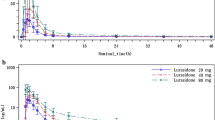
CPZ exhibited multicompartmental pharmacokinetics in most subjects. There was wide between-subject variability in half life (11.05 h), volume of distribution (1215 l), volume of distribution at steady state (642 l) and mean residence time (8.88 h), whereas systemic clearance was somewhat less variable (76.6 l·h−1). All metabolites were present in measurable concentrations in the plasma of 9 of 11 subjects after IV CPZ, whereas free 7-HOCPZ was not detected in the other 2 individuals. With the exception of CPZNO, the biological half lives of the primary metabolites were longer than the half life of CPZ.
After oral administration, the percentage of CPZ reaching the systemic circulation intact (F%) was very low (4–38%) and dose dependant. Moreover, both within-subject and between-subject variances were very high. The maximum plasma concentration (Cmax) and area under the plasma concentration versus time curve extrapolated to infinite time (AUC) showed evidence of nonlinearity, whereas half life did not appear to be dose dependant. These data suggest that the high degree of variability in the pharmacokinetics of CPZ is a result of extensive first pass metabolism rather than variation in half life. The mean AUC for the total conjugates of 7-HOCPZ was about two fold higher than that of the parent drug or any other metabolite. This shows that phase II metabolism plays a very significant role in the disposition of CPZ. As a result, the role of CYP2D6 in the 7-hydroxylation of CPZ cannot be fully assessed without taking phase II metabolism into account.
Similar content being viewed by others
References
Turano P, Turner WJ, Manian AA (1973) Thin-layer chromatography of chlorpromazine metabolites. Attempt to identify each of the metabolites appearing in the blood, urine and feces of chronically medicated schizophrenics. J Chromatogr 75: 277–293
Kleinmann JE, Bigelow LB, Rogol A, Weinberger DR, Nasrallah HA, Wyatt RJ, Gillin JC (1980) A clinical trial of 7-hydroxy-chlorpromazine in chronic schizophrenia. In: Usdin E, Eckert H, Forrest IS (ed) Phenothiazines and structurally related drugs: Basic and clinical studies, Elsevier, Amsterdam New York Oxford, pp 275–278
Nyback H, Sedvall G (1972) Effect of chlorpromazine and some of its metabolites on synthesis and turnover of catecholamines formed from 14C-tyrosine in mouse brain. Psychopharmacologia (Berl) 26: 155–160
Dahl SG (1982) Active metabolites of neuroleptic drugs: Possible contribution to therapeutic and toxic effects. Ther Drug Monit 4: 33–40
Dahl SG, Hjorth M, Hough E (1982) Chlorpromazine, methotrimeprazine and metabolites. Structural changes accompanying the loss of neuroleptic potency by ring sulforxidation. Mol Pharmacol 21: 409–414
Jaworski TJ, Hawes EM, McKay G, Midha KK (1990) The metabolism of chlorpromazine N-oxide in man and dog. Xenobiotica 20: 107–115
Jorgensen A (1986) Metabolism and pharmacokinetics of antipsychotic drugs. In: Bridges JW, Chasseaud LF (eds) Progress in drug metabolism. Taylor and Francis, London Philadelphia
Loo JCK, Midha KK, McGilveray IJ (1980) Pharmacokinetics of chlorpromazine in normal volunteers. Commun Psychopharmacol 4: 121–129
Cooper TB (1978) Plasma level monitoring of antipsychotic drugs. Clin Pharmacokinet 3: 14–38
Inaba T, Jurima M, Mahon WA, Kalow W (1985) In vitro inhibition studies of two isozymes of human liver cytochrome P-450: mephenytoin p-hydroxylase and sparteine monooxygenase. Drug Metab Dispos 13: 443–448
von Bahr C, Spina E, Birgersson C, Ericsson O, Goransson M, Henthorn T, Sjoqvist F (1985) Inhibition of desmethylimipramine 2-hydroxylation by drugs in human liver microsomes. Biochem Pharmacol 34: 2501–2505
Fonne-Pfister R, Meyer UA (1988) Xenobiotic and endobiotic inhibitors of cytochrome P-450dbl function, the target of the debrisoquine/sparteine polymorphism. Biochem Pharmacol 37: 3829–3835
von Bahr C, Movin G, Nordin C, Liden A, Hammerland-Udenaes M, Hedberg A, Ring H, Sjoqvist F (1991) Plasma levels of thioridazine and metabolites are influenced by the debrisoquin hydroxylation phenotype. Clin Pharmacol Ther 49: 234–240
Dahl-Puustinen M-L, Liden A, Alm C, Nordin RN, Bertilsson L (1989) Disposition of perphenazine is related to polymorphic debrisoquin hydroxylation in human beings. Clin Pharmacol Ther 46: 78–81
Meyer JW, Woggon B, Baumann P, Meyer UA (1990) Clinical implication of slow sulphoxidation of thioridazine in a poor metabolizer of the debrisoquine type. Eur J Clin Pharmacol 39: 613–614
Spina E, Ancione M, Di Rosa AE, Meduri M, Caputi AP (1992) Polymorphic debrisoquine oxidation and acute neuroleptic-induced side effects. Eur J Clin Pharmacol 42: 347–348
Speirs CJ, Murray S, Boobis AR, Seddon CE, Davies DS (1986) Quinidine and the identification of drugs whose elimination is impaired in subjects classified as poor metabolizers of debrisoquin. Br J Pharmacol 22: 739–743
Midha KK, Loo JCK, Hubbard JW, Rowe ML, McGilveray IJ (1979) Radioimmunoassay for chlorpromazine in plasma. Clin Chem 25: 166–168
Yeung PKF, Hubbard JW, Cooper JK, Midha KK (1983) A study of the kinetics of chlorpromazine sulfoxide by a specific radioimmunoassay after a single dose of chlorpromazine in healthy volunteers. J Pharmacol Exp Ther 226: 833–838
Yeung PKF, Hubbard JW, Korchinski ED, Midha KK (1987) Radioimmunoassay for the N-oxide metabolite of chlorpromazine in human plasma and its application to a pharmacokinetic study in healthy humans. J Pharm Sci 76: 803–808
Yeung PKF, McKay G, Ramshaw IA, Hubbard JW, Midha KK (1985) A comparison of two radioimmunassays for 7-hydroxy-chlorpromazine: rabbit polyclonal antibodies vs mouse monoclonal antibodies. J Pharmacol Exp Ther 233: 816–822
Midha KK, Hubbard JW, Cooper JK, Gurnsey T, Hawes EM, McKay G, Chakraborty BS, Yeung PKF (1987) Therapeutic monitoring of chlorpromazine IV: comparison of a new high performance liquid chromatographic method with radioimmunoassays for parent drug and some of its major metabolites. Ther Drug Monit 9: 358–365
Ritschel WA (1986) Basic Pharmacokinetics. Drug Intelligence Publications, Hamilton
Chan KK (1982) A simple and integrated method for drug and derived metabolite kinetics. An application of the statistical moment theory. Drug Metab Dispos 10: 474–479
Midha KK, Ormsby ED, Hubbard JW, McKay G, Hawes EM, Gavalas L, McGilveray IJ (1993) Logarithmic transformation in bioequivalence: application with two formulations of perphenazine. J Pharm Sci 82: 138–144
Gibaldi M, Perrier D (1982) Pharmacokinetics. Dekker, New York
Dahl SG, Strandjord RE (1976) Pharmacokinetics of chlorpromazine after single and chronic doses. Clin Pharmacol Ther 21: 437–448
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Yeung, P.K.F., Hubbard, J.W., Korchinski, E.D. et al. Pharmacokinetics of chlorpromazine and key metabolites. Eur J Clin Pharmacol 45, 563–569 (1993). https://doi.org/10.1007/BF00315316
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00315316