Abstract
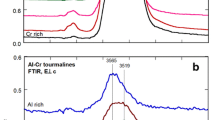
Infrared and electron microprobe analysis of natural tourmalines from the dravite-schorl and elbaite-schorl series were carried out. The infrared study differentiates between OH groups located at the centre of hexagonal rings and those which are placed between hexagonal pillars and are coordinated to two Al ions. The correlation of infrared spectra with chemical composition of tourmalines made possible the assignment of different OH stretching bands to the more frequent octahedral cation associations. The study of the thermal dehydroxylation of tourmalines in air indentified the IR bands corresponding to OH bonded to Fe+2 ions in AlAlFe, AlFeLi or FeFeFe environments. The change in intensity of the OH absorption lines with the sample orientation has permitted the identification of several orientations of the OH bond axes. Electron microprobe analysis of zoned coloured samples has shown that the Fe, Mn distribution is partially ordered in some samples of the elbaite-schorl series.
Similar content being viewed by others
References
Bence AE, Albee AL (1968) Empirical correction factors for the electron microanalysis of silicates and oxides. J Geol 76:382–403
Buerger MJ, Burnham CW, Peacor DR (1962) Assessment of the several structures proposed for tourmaline. Acta Crystallogr 15:583–590
Deer WA, Howie RA, Zussman J (1962) An introduction to the Rock-forming minerals. Longmans, Green and Co. Ltd. London
Foit FF Jr., Rosenberg PE (1977) Coupled substitutions in the tourmaline group. Contr Min Petrol 62:109–127
Gebert W, Zemann J (1965) Messung des Ultrarot-Pleochroismus von Mineralen II. Der Pleochroismus der OH-Streckfrequenz in Turmalin. Neues Jahrb Mineral Monatsh 8:232–235
Gorskaya MG, Frank-Kamenetskaya OV, Rozhdestvenskaya IV, Frank-Kamenetskii VA (1982) Refinement of the crystal structure of Al-rich elbaite, and some aspects of the crystal chemistry of tourmalines. Sov Phys Crystallogr 27:63–66
Hawthorne FC (1983) Quantitative characterization of site occupancies in minerals. Am Mineral 68:287–306
Henry DJ, Guidotti CV (1985) Tourmaline as a petrogenetic indicator mineral: an example from the staurolite-grade metapelites of NW Maine. Am Mineral 70:1–15
Kunitz W (1929) Beiträge zur Kenntnis der magmatischen Assoziationen. I. Die Mischungsreihen in der Turmalin-gruppe und die genetischen Beziehungen zwischen Turmalinen und Glimmern. Chem Erde 4:208–251
Ramberg H (1952) Chemical bonds and distribution of cations in silicates. J Geol 60:331–355
Rausell JA, Sanz J, Fernandez M, Serratosa JM (1979) Distribution of octahedral ions in phlogopites and biotites. In: Mortland MM and Farmer VC (ed) Development in Sedimentology 27. Elsevier, Amsterdam, pp 27–36
Rausell JA, Serratosa JM (1985) Perturbation of v OH infrared frequencies by interlayer cations in homoionic vermiculites. Structural implications. Mineral Petrogr Acta 29:409–423
Rosenberg PE, Foit FF Jr (1979) Synthesis and characterization of alkalii-free tourmaline. Am Mineral 64:180–186
Rossman GR, Mattson SM (1986) Yellow, Mn-rich elbaite with Mn-Ti intervalance charge transfer. Am Mineral 71:599–602
Rouxhet PG (1970) Hydroxyl stretching bands in micas: A quantitative interpretation. Clay Miner 8:375–388
Sanz J, Gonzalez-Carreño T, Gancedo R (1983) On dehydroxylation mechanisms of a biotite in vacuo and in oxygen. Phys Chem Minerals 9:14–18
Sanz J, Stone WEE (1983) NMR study of minerals. III. The distribution of Mg+2 and Fe+2 around the OH groups in micas. J Phys C 16:1271–1281
Serratosa JM, Bradley WF (1958) Determination of the orientation of OH bond axes in layer silicates by infrared absorption. J Phys Chem 62:1164–1167
Shigley JE, Kane RE, Manson DV (1986) A notable Mn-rich gem elbaite tourmaline and its relationship to tsilaisite. Am Mineral 71:1214–1216
Slivko MN (1961) Manganese tourmaline. Int Geol Rev 3:195–210
Strens RGJ (1974) The common chain, ribbon and ring silicates. In: Farmer VC (ed). The infrared spectra of minerals. Mineralogical Society Monography 4, Mineralogical Society. London. pp 305–330
Vedder W (1964) Correlations between infrared spectrum and chemical composition of mica. Am Mineral 49:736–768
Vedder W, McDonald RS (1963) Vibrations of the OH ions in muscovite. J Chem Phys 38:1583–1590
Vedder W, Wilkins RWT (1969) Dehydroxylation and rehydroxylation, oxidation and reduction of micas. Am Mineral 54:482–509
Author information
Authors and Affiliations
Additional information
Paper from the NATO Advanced Study Institute on Physical Properties and Thermodynamic Behaviour of Minerals, Cambridge 1987
Rights and permissions
About this article
Cite this article
Gonzalez-Carreño, T., Fernández, M. & Sanz, J. Infrared and electron microprobe analysis of tourmalines. Phys Chem Minerals 15, 452–460 (1988). https://doi.org/10.1007/BF00311124
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF00311124