Abstract
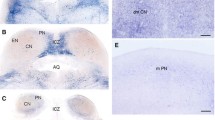
The thalamic reticular nucleus (NRT) is one of the most vulnerable structures to selective neuronal damage both in human cardiac arrest patients and in experimental rodent global cerebral ischemia models. The detailed distribution of neuronal injury within the NRT was examined following 10-min cardiac arrest in the rat with conventional Nissl staining, 45Ca autoradiography and immunocytochemistry of the calcium binding proteins parvalbumin (PV) and calretinin (CR). While Nissl staining was almost unable to show the exact boundary of the nucleus and of the lesion, immunocytochemistry of PV proved to be the most useful index of the exact location and extent of neuronal loss in the NRT after ischemia. Calcium autoradiography was a sensitive method for detecting the lesion, and showed a similar distribution to the loss of PV staining, but did not give optimal spatial resolution. Quantitative analysis of PV staining at 7 days of recirculation demonstrated cell loss restricted to the lateral aspect of the middle segment of the NRT, identical with the distribution of large fusiform neurons in the somatosensory component of the nucleus. CR-positive neurons in the NRT were completely spared, although not all surviving neurons contained CR. These studies provide the first detailed characterization of the distribution of vulnerable neurons within the NRT after experimental ischemia and suggest that immunocytochemistry of PV is a useful tool for quantitative analysis of the lesion for use in further experiments to elucidate the mechanisms of selective vulnerability of the NRT.
Similar content being viewed by others
References
Baimbridge KG, Miller JJ, Parkes CO (1982) Calcium-binding protein distribution in the rat brain. Brain Res 239:519–525
Blomqvist P, Wieloch T (1985) Ischemic brain damage in rats following cardiac arrest using a long-term recovery model. J Cereb Blood Flow Metab 5:420–431
Brierley JB, Graham DI (1984) Hypoxia and vascular disorders of the central nervous system. In: Adams JH, Corsellis JAN, Duchen LW (eds) Greenfield's neuropathology, 4th edn. Wiley-Medical, New York, pp 125–156
Caronna JJ (1986) Neurologic syndromes following cardiac arrest and cardiac bypass surgery. In: Barnett HJM, Stein BM, Mohr JP, Yatsu FM (eds) Stroke: pathophysiology, diagnosis, and management. Churchill Livingstone, New York, pp 747–762
Celio MR, Heizmann CW (1981) Calcium-binding protein parvalbumin as a neuronal marker. Nature 293:300–302
Crick F (1984) Function of the thalamic reticular complex: the searchlight hypothesis. Proc Natl Acad Sci USA 81:4586–4590
Dienel GA (1984) Regional accumulation of calcium in postischemic rat brain. J Neurochem 43:913–925
Freund TF, Buzsaki G, Leon A, Baimbridge KG, Somogyi P (1990) Relationship of neuronal vulnerability and calcium binding protein immunoreactivity in ischemia. Exp Brain Res 83:55–66
Gonzales C, Lin RC, Chesselet MF (1992) Relative sparing of GABAergic interneurons in the striatum of gerbils with ischemia-induced lesions. Neurosci Lett 135:53–58
Heizmann CW, Berchtold MW (1987) Expression of parvalbumin and other Ca2+-binding proteins in normal and tumor cells: a topical review. Cell Calcium 8:1–41
Jones EG (1988) Modern view of cellular thalamic mechanisms. In: Bentivoglio M, Spreafico R (eds) Cellular thalamic mechanisms. Elsevier, Amsterdam, pp 1–22
Kawaguchi Y, Katsumaru H, Kosaka T, Heizmann CW, Hama K (1987) Fast spiking cells in rat hippocamupus (CA1 region) contain the calcium-binding protein parvalbumin. Brain Res 416:369–374
Kawai K, Nitecka L, Ruetzler CA, Nagashima G, Joo F, Mies G, Nowak TS Jr, Saito N, Lohr JM, Klatzo I (1992) Global cerebral ischemia associated with cardiac arrest in the rat. I. Dynamics of early neuronal changes. J Cereb Blood Flow Metab 12:238–249
Kawai K, Nitecka L, Ruetzler CA, Lohr J, Saito N, Joo F, Mies G, Nowak TS Jr, Klatzo I (1992) Dynamics of ischemic injury following global cerebral ischemia in a rat cardiac arrest model. In: Globus MYT, Dietrich WD (eds) The role of neurotransmitters in brain injury. Plenum Press, New York, pp 207–211
Kirino T, Tamura A, Sano K (1984) Delayed neuronal death in the rat hippocampus following transient forebrain ischemia. Acta Neuropathol (Berl) 64:139–147
Koh J-Y, Goldberg MP, Hartley DM, Choi DW (1990) Non-NMDA receptor-mediated neurotoxicity in cortical culture. J Neurosci 10:693–705
Korpatchev WG, Lysenkov SP, Thieliz PS (1982) Modeling clinical death and postresuscitation disease in rats (in Russian). Patol Fiziol Eksp Ter 3:78–80
Mies G, Kawai K, Saito N, Nagashima G, Nowak TS Jr, Klatzo I (1993) Cardiac arrest-induced complete cerebral ischaemia in the rat: dynamics of postischaemic in vivo calcium uptake and protein synthesis. Neurol Res 15:253–263
Minamisawa H, Nordström CH, Smith ML, Siesjö BK (1990) The influence of mild body and brain hypothermia on ischemic brain damage. J Cereb Blood Flow Metab 10:365–374
Nitch C, Scotti A, Sommacat A, Kalt G (1989) GABAergic hippocampal neurons resistant to ischemia-induced neuronal death contain the Ca2+-binding protein parvalbumin. Neurosci Lett 105:263–268
Paxinos G, Watson C (1982) The rat brain in stereotaxic coordinates. Academic Press, Sydney
Ross DT, Duhaime AC (1989) Degeneration of neurons in the thalamic reticular nucleus following transient ischemia due to raised intracranial pressure: excitotoxic degeneration mediated via non-NMDA receptors? Brain Res 501:129–143
Ross DT, Graham DI (1993) Selective loss and selective sparing of neurons in the thalamic reticular nucleus following human cardiac arrest. J Cereb Blood Flow Metab 13:558–567
Shosaku A, Kayama Y, Sumitomo I, Sugitani M, Iwama K (1989) analysis of recurrent inhibitory circuit in rat thalamus: neurophysiology of the thalamic reticular nucleus. Prog Neurobiol 32:77–102
Siesjö BK, Bengtsson F (1989) Calcium fluxes, calcium antagonists, and calcium-related pathology in brain ischemia, hypoglycemia, and spreading depression: a unifying hypothesis. J Cereb Blood Flow Metab 9:127–140
Sloviter RS (1989) Calcium-binding protein (calbindin-D28k) and parvalbumin immunocytochemistry: localization in the rat hippocampus with specific references to the selective vulnerability of hippocampal neurons to seizure activity. J Comp Neurol 280:183–196
Smith ML, Auer RN, Siesjö BK (1984) The density and distribution of ischemic brain injury in the rat following 2–10 min of forebrain ischemia. Acta Neuropathol (Berl) 64:319–332
Spreafico R, Battaglia G, Frassoni C (1991) The reticular thalamic nucleus (RTN) of the rat: cytoarchitectural, Golgi, immunocytochemical, and horseradish peroxidase study. J Comp Neurol 304:478–490
Steriade M, Llinás RR (1988) The functional states of the thalamus and the associated neuronal interplay. Physiol Rev 68: 649–742
Steriade M, Jones EG, Llinás RR (1990) Thalamic oscillations and signaling. Wiley, New York, pp 61–67
Weiss JH, Koh JY, Baimbridge KG, Choi DW (1990) Cortical neurons containing somatostatin- or parvalbumin-like immunoreactivity are atypically vulnerable to excitotoxic injury in vitro. Neurology 40:1288–1292
Winsky L, Nakata H, Martin BM, Jacobowitz DM (1989) Isolation, partial amino acid sequence and immunohistochemical localization of a brain-specific calcium-binding protein. Proc Natl Acad Sci USA 86:10139–10143
Winsky L, Montpied P, Arai R, Martin BM, Jacobowitz DM (1992) Calretinin distribution in the thalamus of the rat: immunohistochemical and in situ hybridization histochemical analyses. Neuroscience 50:181–196
Zeevalk GD, Jacobowitz DM (1992) Ca2+ binding proteins in chick retina and sensitivity to excitotoxicity. Soc Neurosci Abstr 18:1360
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Kawai, K., Nowak, T.S. & Klatzo, I. Loss of parvalbumin immunoreactivity defines selectively vulnerable thalamic reticular nucleus neurons following cardiac arrest in the rat. Acta Neuropathol 89, 262–269 (1995). https://doi.org/10.1007/BF00309342
Received:
Revised:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00309342