Summary
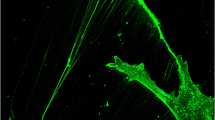
To study the role of phagocytosis in periodontal tissues, internalization of fibronectin-coated latex beads by Gin-1 fibroblast populations was investigated. Demonstration of phagocytosis by internalization of beads was confirmed by immunofluorescence microscopy, electron microscopy, and flow-cytometry. The percent of cells phagocytosing beads measured by flow-cytometry was negligible at 4° and 23°C, but increased to approximately 17% at 37°C. As measured by automated image analysis, the percentage of phagocytosing cells increased linearly from 8 to 22 with increasing fibronectin concentration of the incubation solution from 30 ng to 300 μg/ml. Similar linear increases in the percentage of phagocytosing cells were observed when beads were incubated with cells for periods ranging from 2 h to 2 days. To examine the role of the Arg-Gly-Asp receptor in mediating phagocytosis, fibronectin-coated beads were first coated with either Gly-Arg-Gly-Asp-Ser-Pro or Gly-Arg-Gly-Glu-Ser-Pro peptides at concentrations of 0.125, 0.5, and 1 mg/ml, or with control vehicle, and then incubated with cells. Phagocytosis was completely blocked at 1 mg/ml of the Gly-Arg-Gly-Asp-Ser-Pro peptide, but the Gly-Arg-Gly-Glu-Ser-Pro peptide showed no significant inhibition compared to control values. Blocking antibodies to the cell attachment domain of the fibronectin molecule also reduced the percentage of phagocytosing cells significantly. The data show that these phagocytic assays are sensitive enough to detect the influence of incubation temperature and time, cellular heterogeneity, ligand type, and ligand concentration on the percentage of phagocytosing cells. Further, the mechanisms which determine internalization of fibronectin-coated beads rely in part on the initial binding of ligand to the Arg-Gly-Asp receptor present on fibroblasts.
Similar content being viewed by others
References
Akiyama SK, Yamada KM (1985) Synthetic peptides competitively inhibit both direct binding to fibroblasts and functional assays for the purified cell-binding domain of fibronectin. J Biol Chem 260:10402–10405
Bayreuther K, Rodemann HP, Francz PI, Maier K (1988) Differentiation of fibroblast stem cells. J Cell Sci Suppl 10:115–130
Beertsen W, Everts V (1977) The site of remodelling of collagen in the periodontal ligament of the mouse incisor. Anat Rec 189:479–497
Burleigh MC, Barrett AJ, Lazarus GS (1974) Cathepsin B1. A lysosomal enzyme that degrades native collagen. Biochem J 137:387–398
Dedhar S, Ruoslahti E, Pierschbacher M (1987) A cell surface receptor complex for collagen type I recognizes the Arg-Gly-Asp sequence. J Cell Biol 104:584–593
Deporter DA, Ten Cate AR (1973) Fine structural localization of acid and alkaline phosphatase activity in collagen-containing vesicles of fibroblasts. J Anat 114:457–461
Etherington DJ (1976) Bovine spleen cathepsin B1 and collagenolytic cathepsin. Biochem J 153:199–209
Everts V, Beertsen W (1988) The cellular basis of tooth eruption: the role of collagen phagocytosis. In: The Biological Mechanisms of Tooth Eruption and Root Resorption. Ed. 2. Davidovitch, pp 237–242, EBSCO, Birmingham, Alabama
Farsi JMA, Sodek J, Aubin JE (1985) Fibronectin-independent attachment of human gingival fibroblasts to interstitial and basement membrane collagens. Expl. Cell Res 161:473–483
Grinnell F (1980) Fibroblast receptor for cell substrate adhesion: studies on the interaction of baby hamster kidney cells with latex beads coated by cold-insoluble globulin (plasma fibronectin). J Cell Biol 86:104–112
Klebe RJ, Bentley KL, Schoen R (1981) Adhesion substrates for fibronectin. J Cell Physiol 109:481–488
Limeback HF, Sodek J (1979) Procollagen synthesis and processing in periodontal in vivo and in vitro: a comparative study using slab-gel fluorography. Eur J Biochem 100:541–550
McAbee DA, Grinnell F (1983) Fibronectin-mediated binding and phagocytosis of polystyrene latex beads by baby hamster kidney cells. J Cell Biol 97:1515–1523
McAbee DD, Grinnell F (1985) Binding and phagocytosis of fibronectin-coated beads by BHK cells: Receptor specificity and dynamics. J Cell Physiol 124:240–246
McCulloch CAG, Barghava U, Melcher AH (1988) Cell death and the regulation of populations of cells in the periodontal ligament. Cell Tissue Res 255:129–138
Melcher AH, Chan J (1981) Phagocytosis and digestion of collagen by gingival fibroblasts in vivo: A study of serial sections. J Ultrastruct Res 77:1–36
Murphy G, Reynolds JJ (1984) Current views of collagen degradation. Progress towards understanding the resorption of connective tissues. Bioessays 2:55–60
Narayanan AS, Page RC (1983) Connective tissues of the periodontium: A summary of current work. Collagen Rel Res 3:33–64
Nemeth E, McCulloch CAG, Melcher AH (1989) Coordinated regulation of endothelial and fibroblast cell proliferation and matrix synthesis in periodontal ligament adjacent to appositional and resorptive bone surfaces. Anat Rec 223:368–375
Pierschbacher MD, Hayman EG, Ruoslahti E (1981) Location of the cell-attachment site in fibronectin with monoclonal antibodies and proteolytic fragments of the molecule. Cell 26:259–267
Pitaru S, Aubin JE, Bhargava U, Melcher AH (1987) Immunoelectron microscopic studies on the distributions of fibronectin and actin in a cellular dense connective tissue: the periodontal ligament of the rat. J Periodont Res 22:64–74
Ruoslahti E, Pierschbacher M (1987) New perspectives in cell adhesion: RGD and integrins. Science 238:491–497
Schor SL, Schor AM (1987) Clonal heterogeneity in fibroblast phenotype: Implications for the control of epithelial-mesenchymal interactions. Bioessays 7:200–204
Sodek J (1977) A comparison of the rates of synthesis and turnover of collagen and non-collagen proteins in adult rat periodontal tissues and skin using a microassays. Arch Oral Biol 22:655–665
Svoboda ELA, Melcher AH, Brunette AH (1979) Stereological study of collagen phagocytosis by cultured periodontal ligament fibroblasts: time course and effect of deficient culture medium. J Ultrastruct Res 68:195–208
Vignery A, Baron R (1980) Dynamic histomorphometry of alveolar bone remodelling in the adult rat. Anat Rec 196:191–200
Wagner D, Hynes RO (1982) Fibronectin-coated beads are endocytosed by cells and align with microfilment bundles. Exp Cell Res 140:373–381
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
McKeown, M., Knowles, G. & McCulloch, C.A.G. Role of the cellular attachment domain of fibronectin in the phagocytosis of beads by human gingival fibroblasts in vitro. Cell Tissue Res 262, 523–530 (1990). https://doi.org/10.1007/BF00305249
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00305249