Abstract
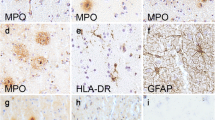
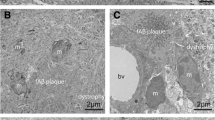
One of the major histopathological lesions in brains of patients with dementia of the Alzheimer type (DAT) is the senile plaque. Although previous studies have shown that senile plaques are often accompanied by microglial cells, the role of these cells in DAT pathology is still unclear. In an immunohistochemical and immuno-electron microscopical analysis of DAT and control brain tissues we addressed this issue using two monoclonal antibodies (mAbs KP1 and 25F9) directed against lysosomal antigens in monocytes and macrophages. Whereas KP1 stained lysosomes in both resting and activated microglial cells, 25F9-staining was predominantly found in lysosomes of activated microglial cells in classic senile plaques. The number and size of 25F9-positive lysosomes in activated microglial cells was increased compared to 25F9-staining in unaffected areas in DAT and control sections. We conclude that mAb 25F9 is a unique and useful lysosomal marker, with a higher specificity than other known markers, for activated microglial cells associated with classic, but not with diffuse, senile plaques.
Similar content being viewed by others
References
Abraham CR, Selkoe DJ, Potter H (1988) Immunochemical identification of the serine protease inhibitor alpha-1-antichymotrypsin in the brain amyloid deposits of Alzheimer's disease. Cell 52: 487–501
Akiyama H, McGeer PL (1990) Brain microglia constitutively express β-2-integrins. J Neuroimmunol 30: 81–93
Akiyama H, Kawamata T, Yamada T, Tooyama I, Ishii T, McGeer PL (1993) Expression of intercellular adhesion molecule (ICAM)-1 by a subset of astrocytes in Alzheimer's disease and some other degenerative neurological disorders. Acta Neuropathol 85: 628–634
Cras P, Gheuens J, Lübke U, Boons J, Vandermeeren M, van Heuverswijn H, Martin J-J (1990) A monoclonal antibody raised against Alzheimer cortex that specifically recognizes a subpopulation of microglial cells. J Histochem Cytochem 38: 1201–1207
Cras P, Kawai M, Siedlak S, Mulvihill P, Gambetti P, Lowery D, Gonzalez-DeWhitt P, Greenberg B, Perry G (1990) Neuronal and microglial involvement in β-amyloid protein deposition in Alzheimer's disease. Am J Pathol 137: 241–246
Dickson DW, Farlo J, Davies P, Crystal H, Fuld P, Yen S-H (1988) Alzheimer's disease. A double-labeling immunobisto-chemical study of senile plaques. Am J Pathol 132: 86–101
Dickson DW, Lee SC, Mattiace LA, Yen SC, Brosnan C (1993) Microglia and cytokines in neurological disease, with special reference to AIDS and Alzheimer's disease. Glia 7: 75–83
Eikelenboom P, Stam FC (1982) Immunoglobulins and complement factors in senile plaques. An immunohistochemical study. Acta Neuropathol (Berl) 57: 239–242
Esiri MM, Morris CS (1991) Immunocytochemical study of macrophages and microglial cells and extracellular matrix components in human CNS disease J Neurol Sci 101: 59–72
Gardella JE, Gorgone GA, Munoz PC, Ghiso J, Frangione B, Gorevic PD (1992) β-Protein precursor expression in human platelets and a megakaryocyte cell line. Lab Invest 67: 303–313
Glenner GG, Wong CW (1984) Alzheimer's disease and Down's syndrome sharing of a unique cerebrovascular amyloid fibril protein. Biochem Biophys Res Commun 122: 1131–1135
Graeber MB, Streit WJ, Büringer D, Sparks L, Kreutzberg GW (1992) Ultrastructural location of major histocompatibility complex (MHC) class II positive perivascular cells in histologically normal human brain. J Neuropathol Exp Neurol 51: 303–311
Haass C, Hung AY, Selkoe DJ (1991) Processing of β-amyloid precursor protein in microglia and astrocytes favors an internal localization. over constitutive secretion. J Neurosci 11: 3783–3793
Haass C, Schlossmacher MG, Hung AY, Vigo-Pelfrey C, Mellon A, Ostaszewski BL, Lieberburg I, Koo EH, Schenk D, Teplow DB, Selkoe DJ (1992) Amyloid β-peptide is produced by cultured cells during normal metabolism. Nature 359: 322–325
Haga S, Akai K, Ishii T (1989) Demonstration of microglial cells in and around senile (neuritic) plaques in the Alzheimer brain. Acta Neuropathol 77: 569–575
Hickey WF, Kimura H (1988) Perivascular microglial cells of the CNS are bone-marrow derived and present antigen in vivo. Science 239: 290–292
Itagaki S, McGeer PL, Akiyama H, Zhu S, Selkoe D (1989) Relationship of microglia and astrocytes to amyloid deposits of Alzheimer disease. J Neuroimmunol 24: 173–182
Kang J, Lemaire H, Unterbeck A, Salbaum JM, Masters CL, Grzeschik K, Multhaup G, Beyreuther K, Müller-Hill B (1987) The precursor of Alzheimer's disease amyloid A4 protein resembles a cell-surface receptor. Nature 325: 733–736
Khachaturian ZS (1985) Diagnosis of Alzheimer's disease. Arch Neurol 42: 1097–1104
König G, Mönning U, Czech C, Prior R, Banati R, Schreiter-Gasser U, Bauer J, Masters CL, Beyreuther K (1992) Identification and differential expression of a novel alternative splice isoform of the βA4 amyloid precursor protein (βAPP) mRNA in leukocytes and brain microglial cells. J Biol Chem 267: 10804–10809
Ling E-A, Wong W-C (1993) The origin and nature of ramified and amoeboid microglia: a historical review and current concepts. Glia 7: 9–18
Maat-Schieman MLC, Rozemuller JM, Duinen SG van, Haan J, Eikelenboom P, Roos RAC (1994) Microglia in diffuse plaques in hereditary cerebral hemorrhage with amyloidosis (Dutch). An immunohistochemical study. J Neuropathol Exp Neurol 53: 483–491
Mannoji H, Yeger H, Becker LE (1986) A specific histochemical marker (lectin Ricinus communis agglutinin-1) for normal human microglia, and application to routine histopathology. Acta Neuropathol (Berl) 71: 341–343
Mattiace LA, Davies P, Dickson DW (1990) Detection of HLA-DR on microglia in the human brain is a function of both clinical and technical factors. Am J Pathol 136: 1101–1114
McGeer PL, Itagaki S, Tago H, McGeer EG (1987) Reactive microglia in patients with senile dementia of the Alzheimer type are positive for the histocompatibility glycoprotein HLA-DR. Neurosci Lett 79: 195–200
Mirra SS, Heyman A, McKeel D, Sumi SM, Crain BJ, Brownlee LM, Vogel FS, Hughes JP, Belle G van, Berg L (1991) The consortium to establish a registry for Alzheimer's Disease (CERAD). Part II. Standardization of the neuropathologic assessment of Alzheimer's disease. Neurology 41: 479–486
Peress NS; Fleit HB, Perillo E, Kuljis R, Pezzullo C (1993) Identification of FcγRI, II and III on normal human brain ramified microglia and on microglia in senile plaques in Alzheimer's disease. J Neuroimmunol 48: 71–80
Perlmutter LS, Scott SA, Barrón E, Chui HC (1992) MHC class II-positive microglia in human brain: association with Alzheimer lesions. J Neurosci Res 33: 549–558
Probst A, Brunnschweiler H, Lautenschlager C, Ulrich J (1987) A special type of senile plaque, possibly an initial stage. Acta Neuropathol (Berl) 74: 133–141
Pulford KAF, Rigney EM, Micklem KJ, Jones M, Stross WP, Gatter KC, Mason DY (1989) KP1: a new monoclonal antibody that detects a monocyte/macrophage associated antigen in routinely processed tissue sections. J Clin Pathol 42: 414–421
Rozemuller JM, Eikelenboom P, Pals ST, Stam FC (1989) Microglial cells around amyloid plaques in Alzheimer's disease express leukocyte adhesion molecules of the LFA-1 family. Neurosci Lett 101: 288–292
Rozemuller JM, Eikelenboom P, Stam FC, Beyreuther K, Masters CL (1989) A4 protein in Alzheimer's disease: primary and secondary cellular events in extracellular amyloid deposition. J Neuropathol Exp Neurol 48: 674–691
Rozemuller JM, Valk P van der, Eikelenboom P (1992) Activated microglia and cerebral amyloid deposits in Alzheimer's disease. Res Immunol 143: 646–649
Rozemuller JM, Roos RAC, Bots GTAM, Kamphorst W, Eikelenboom P, Van Nostrand WE (1993) Distribution of β/A4 protein and amyloid precursor protein in hereditary cerebral hemorrhage with amyloidosis-Dutch type and Alzheimer's disease. Am J Pathol 142: 1449–1457
Scott SA, Johnson SA, Zarow C, Perlmutter LS (1993) Inability to detect beta-amyloid protein precursor mRNA in Alzheimer plaque-associated microglia. Exp Neurol 121: 113–118
Shaffer LM, Dority MD, Younkin SG, Brunden KR (1994) Removal of liquid-phase and substrate-bound amyloid β peptide (Aβ) by microglia and other CNS cell types (abstract). Neurobiol Aging 15: S152
Shoji M, Hirai S, Yamaguchi H, Harigaya Y, Kawarabayashi T (1990) Amyloid β-protein precursor accumulates in dystrophic neurites of senile plaques in Alzheimer-type dementia. Brain Res 512: 164–168
Shoji M, Hirai S, Yamaguchi H, Harigaya Y, Ishiguro K, Matsubara E (1991) Alpha-1-antichymotrypsin is present in diffuse senile plaques. Am J Pathol 138: 247–257
Strauss S, Bauer J, Ganter U, Jonas U, Berger M, Volk B (1992) Detection of interleukin-6 and α-2-macroglobulin immunoreactivity in cortex and hippocampus of Alzheimer's disease patients. Lab Invest 66: 223–230
Tagliavini F, Ghiso J, Timmers WF, Giaccone G, Bugiani O, Frangione B (1990) Coexistence of Alzheimer's amyloid precursor protein and amyloid protein in cerebral vessel walls. Lab Invest 62: 761–767
Tamaoka A, Kalaria RN, Lieberburg I, Selkoe DJ (1992) Identification of a stable fragment of the Alzheimer amyloid precursor containing the β-protein in brain microvessels. Proc Natl Acad Sci USA 89: 1345–1349
Theele DP, Streit WJ (1993) A chronicle of microglial ontogeny. Glia 7: 5–8
Tooyama I, Kimura H, Akiyama H, McGeer PL (1990) Reactive microglia express class I and class II major histocompatibility complex antigens in Alzheimer's disease. Brain Res 523: 273–280
Ulvestad E, Williams K, Mork S, Antel J, Nyland H (1994) Phenotypic differences between human monocytes/macrophages and microglial cells studied in situ and in vitro. J Neuropathol Exp Neurol 53: 492–501
Van Nostrand WE, Wagner SL, Suzuki M, Choi BH, Farrow JS, Geddes JW, Cotman CW, Cunningham DD (1989) Protease nexin-II, a potent anti-chymotrypsin, shows identity to amyloid β-protein precursor. Nature 341: 546–549
Verbeek MM, Otte-Höller I, Wesseling P, Ruiter DJ, Waal RMW de (1994) Induction of α-smooth muscle actin expression in cultured human brain pericytes by TGFβ1. Am J Pathol 144: 372–382
Verbeek MM, Otte-Höller I, Westphal JR, Wesseling P, Ruiter DJ, Waal RMW de (1994) Accumulation of intercellular adhesion molecule-1 in senile plaques in brain tissue of patients with Alzheimer's disease. Am J Pathol 144: 104–116
Wegiel J, Wisniewski HM (1990) The complex of microglial cells and amyloid star in three-dimensional reconstruction. Acta Neuropathol 81: 116–124
Wisniewski HM, Wegiel J, Wang KC, Kujawa M, Lach B (1989) Ultrastructural studies of the cells forming amyloid fibers in classical plaques. Can J Neurol Sci 16: 535–542
Wisniewski HM, Wegiel J, Wang KC, Lach B (1992) Ultrastructural studies of the cells forming amyloid in the cortical vessel wall in Alzheimer's disease. Acta Neuropathol 84: 117–127
Zwadlo G, Bröcker E, Bassewitz D von, Feige U, Sorg C (1985) A monoclonal antibody to a differentiation antigen present on mature human macrophages and absent from monocytes. J Immunol 134: 1487–1492
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Verbeek, M.M., Otte-Höller, I., Wesseling, P. et al. A lysosomal marker for activated microglial cells involved in Alzheimer classic senile plaques. Acta Neuropathol 90, 493–503 (1995). https://doi.org/10.1007/BF00294811
Received:
Revised:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00294811