Abstract
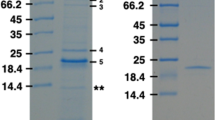
Oligomeric porin of the phototrophic bacterium Rhodopseudomonas blastica DSM 2131 was obtained from cell envelopes by differential temperature extraction in the presence of detergent and salt. The isolated porin exhibited strong porin activity after reconstitution into lipid bilayer membranes. The effective channel diameter for the trimer was estimated as 1.5 nm from single channel conductance measurements in the presence of 1 M KCl. Moderate cation-selectivity was observed. Oligomeric porin migrated as a single band (apparent molecular weight 81 kDa) on sodium dodecyl sulfate polyacrylamide gelelectrophoresis when solubilized below 70 °C. The oligomers were converted into monomers on heating to 70 °C or above forming two bands with apparent molecular weight of 36 kDa and 35 kDa. The oligomer was not sensitive to EDTA. Its molecular weight was determined to be 119.3 kDa by analytical ultracentrifugation. The isoelectric point was 5.7. Circular dichroism data indicated a high content of β-sheet structure. Gasphase sequencing of the N-terminal residues revealed the sequence: NH2-Glu-Ile-Ser-Leu-Asn-Gly-Tyr-Gly-Arg-Phe. Crystals with a maximal side length of 300 μm and diffracting to 0.32 nm resolution were obtained with the porin oligomer in the presence of C8E4 and 1,2,3-heptanetriol by using the vapor phase equilibration technique.
Similar content being viewed by others
Abbreviations
- C8E4:
-
n-octyl tetraoxyethylene
- Mr :
-
apparent molecular weight
- Octyl-POE:
-
n-octyl polyoxyethylene
- LDAO:
-
N,N-dimethyl dodecyl aminoxide
- LPS:
-
lipopolysaccharide
- PAGE:
-
polyacrylamide gel-electrophoresis
- PEG:
-
polyethylene glycol
References
Benz R (1985) Porin from bacterial and mitochondrial outer membranes. Crit Rev Biochem 19: 145–190
Benz R (1988) Structure and function of porins from gram-negative bacteria. Anu Rev Microbiol 42: 359–393
Benz R, Bauer K (1988) Permeation of hydrophilic molecules through the outer membrane of gram-negative bacteria. Eur J Biochem 176: 1–19
Benz R, Janko K, Boos W, Läuger P (1978) Formation of large, ion-permeable membrane channels by the matrix protein (porin) of Escherichia coli. Biochim Biophys Acta 511: 305–319
Benz R, Janko K, Läuger P (1979) Ionic selective of pores by the matrix protein (porin) of Escherichia coli. Biochim Biophys Acta 551: 238–247
Benz R, Woitzik D, Flammann HT, Weckesser J (1987) Pore forming activity of the major outer membrane protein of Rhodobacter capsulatus in lipid bilayer membranes. Arch Microbiol 148: 226–230
Cowan SW, Schirmer T, Rummel G, Steiert M, Ghosh R, Pauptit RA, Jansonius JN, Rosenbusch JP (1992) Crystal structures explain functional properties of two E. coli porins. Nature 358: 727–733
Deal CD, Kaplan S (1983) Physical and chemical characterization of the outer membrane protein of Rhodopseudomonas sphaervides. J Biol Chem, 258: 6530–6536
Diedrich DL, Stein MA, Schnaitman CA (1990) Association of Escherichia coli K-12 OmpF trimers with rough and smooth lipopolysaccharides. J Bacteriol 172: 5307–5311
Drews G (1965) Die Isolierung schwefelfreier Purpurbakterien. Zbl Bakt Hyg Orig Suppl 1: 170–178
Eckersley K, Dow CS (1980) Rhodopseudomonas blastica sp. nov.: a member of the Rhodospirillaceae. J Gen Microbiol 119: 465–473
Flammann HT, Weckesser J (1984a) Porin isolated from the cell envelope of Rhodopseudomonas capsulata. J Bacteriol 159: 410–412
Flammann HT, Weckesser J (1984b) Characterization of the cell wall and outer membrane of Rhodopseumonas capsulata. J Bacteriol 159: 191–198
Furukawa H, Yamada H, Mizushima S (1979) Interaction of bacteriophage T4 with reconstituted cell envelopes of Escherichia coli K 12. J Bacteriol 140: 1071–1080
Garavito RM, Rosenbusch JP (1980) Three-dimensional crystals of an integral membrane protein: an initial X-ray. J Cell Biol 86: 327–329
Hancock REW, Carey AM (1979) Outer membrane of Pseudomonas aeruginosa: heat- and 2- mercaptoethanol-modifiable proteins. J Bacteriol 140: 902–910
Holzenburg A, Engel A, Kessler R, Manz HJ, Lustig A, Aebi U (1989) Rapid isolation of Omp F porin-LPS complexes suitable for structure-function studies. Biochemistry 28: 4187–4193
Jap BK, Walian PJ (1990) Biophysics of the structure and function of porins. Q Rev Biophys 23: 367–403
Kreusch A, Weiss MS, Welte W, Weckesser J, Schulz GE (1991) Crystals of an integral membrane protein diffracting to 1.8 A resolution. J Mol Biol 217: 9–10
Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head bacteriophage T4. Nature 227: 680–685
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193: 265–275
Lugtenberg B, Meijers J, Peters R, van derHock P (1975) Electrophoretic resolution of the ‘major outer membrane protein of Escherichia coli K. 12 into four bands. FEBS Lett 58: 254–258
Michel H (1982) Three-dimensional crystals of a membrane protein complex: the photosynthetic reaction centre from Rhodopseudomonas viridis. J Mol Biol 158: 567–572
Nakae T, Ishii J, Tokunaga M (1979) Subunit structure of functional porin oligomers that form permeability channels in the outer membrane of Escherichia coli. J Biol Chem 254: 1457–1461
Nakamura K, Mizushima S (1976) Effects of heating in dodecyl sulfate solution on the conformation and electrophoretic mobility of isolated major outer membrane proteins from Escherichia coli K-12. J Biochem 88: 1411–1422
Nestel U, Wacker T, Woitzik D, Weckesser J, Kreutz W, Welte W (1989) Crystallization and preliminary X-ray analysis of porin from Rhodobacter capsulatus. FEBS Lett 242: 405–408
Nikaido H, Vaara M (1985) Molecular basis of bacterial outer memebrane premeability. Microbiol Rev 49: 1–32
O'Farrell PH (1975) High resolution two-dimensional electrophoress of proteins. J Biol Chem 150: 4007–4021
Overbeeke N, vanScharrenburg G, Lugtenberg B (1980) Antigenic relationships between pore proteins of Escherichia coli K12. Eur J Biochem 110: 247–254
Reynolds JA, Tanford C (1970) The gross conformation of protein-sodium dodecyl sulfate complexes. J Biol Chem 245: 5161–5165
Rocque WJ, McGroarty EJ (1989) Isolation and preliminary characterization of wild-type OmpC dimers from Escherichia coli K12. Biochemistry 28: 3738–3743
Rocque WJ, Coughlin RT, McGroarty EJ (1987) Lipopolysaccharide tightly bound to porin monomers and trimers from Escherichia coli K12. J Bacteriol 169: 4003–4010
Rosenbusch JP (1974) Characterization of the major envelope protein from Escherichia coli. Regular arrangement on the peptidoglycan and unusual dodecyl sulfate binding. J Biol Chem 249: 8019–8029
Schindler HG, Rosenbusch JP (1978) Matrix porin from Escherichia coli outer membranes forms voltage-controlled channels in lipid bilayers. Proc Natl Acad Sci USA 75: 3751–3755
Schnaitman CA (1971) Solubilization of the cytoplasmic membrane of Escherichia coli by Triton X-100. J Bacteriol 108: 545–552
Spiess M, Hauser H, Rosenbusch JP, Semenza G (1981) Hydrodynamic properties of phospholipid vesicles and of sucrose Isomaltose-phospholipid vesicles. J Biol Chem 256: 8977–8982
Tegtmeyer B, Weckesser J, Mayer H, Imhoff JF (1985) Chemical composition of the lipopolysaccharides of Rhodobacter sulfidophilus, Rhodopseudomonas acidophila und Rhodopseudomonas blastica. Arch Microbiol 143: 32–36
Tokunaga M, Tokunaga H, Okajima Y, Makae T (1979) characterization of porins from the outer membrane of Salmonella typhimurium. 2. Physical properties of the functional oligomeric aggregates. Eur J Biochem 95: 441–448
Tsai C, Frash CE (1982) A sensitive silver staining detecting lipopolysaccharides in polyacrylamid gels. Anal Biochem 119: 115–119
Weckesser J, Zalman S, Nikaido H (1984) Porin from Rhodopseudomonas sphaeroides. J Bacteriol 159: 199–205
Weiss MS, Kreusch A, Schiltz E, Nestel U, Welte W, Weckesser J, Schulz GE (1991a). The structure ofporin from Rhodobacter capsulatus at 1.8 A resolution. FEBS Lett 280: 379–382
Weiss MS, Abele U, Weckesser J, Welte W, Schiltz E, Schulz GE (1991b) Molecular architecture and electrostatic properties of a bacterial porin. Science 254: 1627–1630
Woitzik D (1989) Zellwandproteine von Rhodobacter capsulatus 37b4, Rhodobacter sphaeroides ATCC17023 und Thiobacillus versutus IFO14567. Doctoral thesis, Freiburg i. Br.
Woitzik D, Benz R, Lustig A, Weckesser J (1989) Porin from Thiobacillus versutus. FEMS Microbiol Lett 65: 319–322
Woitzik D, Weckesser J, Benz R, Stevanovic S, Jung G, Rosenbusch JP (1990) Porin of Rhodobacter capsulatus: biochemical and functional characterization. Z Naturforsch 45c: 576–582
Zalman LS, Nikaido H (1985) Dimeric porin from Paracoccus dentrificans. J Bacteriol 162: 430–433
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Butz, S., Benz, R., Wacker, T. et al. Biochemical characterization and crystallization of porin from Rhodopseudomonas blastica . Arch. Microbiol. 159, 301–307 (1993). https://doi.org/10.1007/BF00290911
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00290911