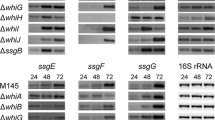
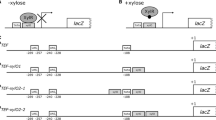
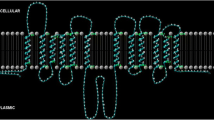
Abstract
Promoters that control gene expression in Saccharomyces cerevisiae only in a sporulation-specific manner have previously been isolated from a genomic yeast DNA library fused to a promoterless Escherichia coli lacZ gene. Two novel sporulation-specific genes, SPS18 and SPS19, were isolated using this technique. These genes are divergently controlled by the same promoter but with SPS18 expressed at four times the level of SPS19. Deletion analysis has shown that the promoter elements that exert sporulation control on each of the genes overlap, having a common 25 bp sequence located within the intergenic region. SPS18 encodes a 34-KDa protein of 300 amino acids that contains a putative zinc-binding domain and a region of highly basic residues that could target the protein to the nucleus. SPS19 encodes a 31-KDa protein of 295 amino acids, which has a peroxisomal targeting signal (SKL) at its C terminus; this protein belongs to the family of non-metallo short-chain alcohol dehydrogenases. A null mutation deleting the intergenic promoter prevented expression of both genes, and when homozygous in diploids, reduced the extent of sporulation four-fold; the spores that did form were viable, but failed to become resistant to ether, and were more sensitive to lytic enzymes. This phenotype reflects a defect in spore wall maturation, indicating that the product of at least one of the genes functions during the process of spore wall formation. Therefore these genes belong to the class of late sporulation-specific genes that are sequentially activated during the process of meiosis and spore formation.
Similar content being viewed by others
References
Berben G, Dumont J, Gilliquet V, Bolle P-A, Hilger F (1991) The YDp plasmids: a uniform set of vectors bearing versatile gene disruption cassettes for Saccharomyces cerevisiae. Yeast 7: 475–477
Briza P, Winkler G, Kalchhauser H, Breitenbach M (1986) Dityrosine is a prominent component of the yeast ascospore wall. A proof of its structure. J Biol Chem 261: 4288–4294
Briza P, Breitenbach M, Ellinger A, Segall J (1990) Isolation of two developmentally regulated genes involved in spore wall maturation in Saccharomyces cerevisiae. Genes Dev 4: 1775–1789
Coe JG, Murray LE, Kennedy CJ, Dawes IW (1992) Isolation and characterization of sporulation-specific promoters in the yeast Saccharomyces cerevisiae. Mol Microbiol 6: 75–81
Cunningham TS, Cooper TG (1991) Expression of the DAL80 gene, whose product is homologous to the GATA factors and is a negative regulator of multiple nitrogen catabolic genes in Saccharomyces cerevisiae, is sensitive to nitrogen catabolite repression. Mol Cell Biol 11: 6205–6215
Dawes IW (1982) Genetic control and gene expression during meiosis and sporulation in Saccharomyces cerevisiae. In: Spencer JFT, Spencer DM, Smith ARW (eds) Yeast genetics: fundamental and applied aspects. Springer-Verlag, New York, pp 29–64
Dawes IW, Hardie ID (1974) Selective killing of vegetative cells in sporulated yeast cultures by exposure to diethyl ether. Mol Gen Genet 131: 281–289
Dickinson JR (1988) The metabolism of sporulation in yeast. Microbiol Sci 5: 121–123
Dickinson JR, Dawes IW, Boyd AJ, Baxter RL (1983) 13CNMR studies of acetate metabolism during sporulation of Saccharomyces cerevisiae. Proc Natl Acad Sci USA 80: 5847–5851
Drebot MA, Johnston GC, Singer RA (1987) A yeast mutant conditionally defective only for reentry into the mitotic cell cycle from stationary phase. Proc Natl Acad Sci USA 84: 7948–7952
Einerhand AWC, Voorn-Brower MM, Erdmann R, Kunau W-H, Tabak HF (1991) Regulation of transcriptional of the gene coding for peroxisomal 3-oxoacyl-CoA thiolase of Saccharomyces cerevisiae. Eur J Biochem 200: 113–122
Esposito RE, Klapholz S (1981) Meiosis and ascospore development. In: Strathern JN, Jones EW, Broach JR (eds) The molecular biology of the yeast Saccharomyces: life cycle and inheritance. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, pp 211–287
Gould SJ, Keller G-A, Hosken N, Wilkinson J, Subramani S (1989) A conserved tripeptide sorts proteins to peroxisomes. J Cell Biol 108: 1657–1664
Henikoff S, Cohen EH (1984) Sequences responsible for transcription termination on a gene segment in Saccharomyces cerevisiae. Mol Cell Biol 4: 1515–1520
Hiltunen JK, Wenzel B, Beyer A, Erdmann R, Kunau WH (1992) Peroxisoml multifunctional β-oxidation protein of Saccharomyces cerevisiae. Molecular analysis of the FOX2 gene and gene product. J. Biol Chem 267: 6646–6653
Hirose A, Kamijo K, Osumi T, Hashimoto T, Mizugaki M (1990) cDNA cloning of rat liver 2,4-dienoyl-CoA reductase. Biochim Biophys Acta 1049: 346–349
Holaway BL, Kao G, Finn MC, Clancy MJ (1987) Transcriptional regulation of sporulation genes in yeast. Mol Gen Genet 210: 449–459
Illingworth RF, Rose AH, Beckett A (1973) Changes in the lipid composition and fine structure of Saccharomyces cerevisiae during ascus formation. J Bacteriol 113: 373–386
Irie S, Doi S, Yorifuji T, Takagi M, Yano K (1987) Nucleotide sequencing and characterization of the genes encoding benzene oxidation enzymes of Pseudomonas putida. J Bacteriol 169: 5174–5179
Jörnvall H, von Bohr-Lindström H, Jany K-D, Ulmer W, Fröschle M (1984) Extended superfamily of short alcohol-polyol-sugar dehydrogenases: structural similarity between glucose and ribitol dehydrogenases. FEBS Lett 165: 190–196
Klapholz S, Waddell CS, Esposito RE (1985) The role of the SP011 gene in meiotic recombination in yeast. Genetics 110: 187–216
Köhrer K, Domdey H (1991) Preparation of high molecular weight RNA. Methods Enzymol 194: 402–405
Kuenzel EA, Mulligan JA, Sommercorn J, Krebs EG (1987) Substrate specificity determinants for casein kinase II as deduced from studies with synthetic peptides. J Biol Chem 262: 9136–9140
Lampel KA, Uratani B, Chaudhry GR, Ramaley RF, Rudikoff S (1986) Characterization of the developmentally regulated Bacillus subtilis glucose dehydrogenase gene. J Bacteriol 166: 238–243
Law DTS, Segall J (1988) The SPS100 gene of Saccharomyces cerevisiae is activated late in the sporulation process and contributes to spore wall maturation. Mol Cell Biol 8: 912–922
Lee M-E, Temizer DH, Clifford JA, Quartermous T (1991) Cloning of the GATA-binding protein that regulates endothelin-1 gene expression in endothelial cells. J Biol Chem 266: 16188–16192
Malavasic MJ, Elder RT (1990) Complementary transcripts from two genes necessary for normal meiosis in the yeast Saccharomyces cerevisiae. Mol Cell Biol 10: 2809–2819
Marekov L, Krook M, Jörnvall H (1990) Prokaryotic 20β-hydroxysteroid dehydrogenase is an enzyme of the ‘short-chain, nonmetalloenzyme’ alcohol dehydrogenase type. FEBS Lett 266: 51–54
Marmorstein R, Carey M, Ptashne M, Harrison SC (1992) DNA recognition by GAL4: structure of a protein-DNA complex. Nature 356: 408–414
Mazo AM, Huang DH, Mozer BA, Dawid IB (1990) The trithorax gene, a trans-acting regulator of the bithorax complex in Drosophila encodes a protein with zinc-binding domains. Proc Natl Acad Sci USA 87: 2112–2116
Myers AM, Tzagoloff A, Kinney DM, Lusty CJ (1986) Yeast shuttle and integrative vectors with multiple cloning sites suitable for construction of lacZ fusions. Gene 45: 299–310
Percival-Smith A, Segall J (1986) Characterisation and mutational analysis of a cluster of three genes expressed preferentially during sporulation of Saccharomyces cerevisiae. Mol Cell Biol 6: 2443–2451
Rose MD, Novick P, Thomas JH, Botstein D, Fink GR (1987) A Saccharomyces cerevisiae genomic plasmid bank based on a centromere-containing shuttle vector. Gene 60: 237–243
Silver PA (1991) How proteins enter the nucleus. Cell 64: 489–497
Van Houten JV, Newlon CS (1990) Mutational analysis of the consensus sequence of a replication origin from yeast chromosome III. Mol Cell Biol 10: 3917–3925
Uerdier JM (1990) Regulatory DNA binding proteins in yeast: an overview. Yeast 6: 271–297
Wang H-T, Frackman S, Kowalisyn J, Esposito RE, Elder R (1987) Developmental regulation of SP013, a gene required for separation of homologous chromosomes at meiosis I. Mol Cell Biol 7: 1425–1435
Weir-Thompson EM, Dawes IW (1984) Developmental changes in translatable RNA species associated with meiosis and spore formation in Saccharomyces cerevisiae. Mol Cell Biol 4: 695–702
Woodgett JR, Gould KL, Hunter T (1986) Substrate specificity of protein kinease C. Use of synthetic peptides corresponding to physiological sites as probes for substrate recognition requirements. Eur J Biochem 161: 177–184
Wright JF, Ajam N, Dawes IW (1981) Nature and timing of some sporulation-specific protein changes in Saccharomyces cerevisiae. Mol Cell Biol 1: 910–918
Yamamoto-Otake H, Koyama Y, Horiuchi T, Nakano E (1991) Cloning, sequencing, and expression of the N-acyl-d-mannosamine dehydrogenase gene from Flavobacterium sp. strain 141-8 in Escherichia coli. Appl Environ Microbiol 57: 1418–1422
Author information
Authors and Affiliations
Additional information
Communicated by B. Kilbey
Rights and permissions
About this article
Cite this article
Coe, J.G.S., Murray, L.E. & Dawes, I.W. Identification of a sporulation-specific promoter regulating divergent transcription of two novel sporulation genes in Saccharomyces cerevisiae . Molec. Gen. Genet. 244, 661–672 (1994). https://doi.org/10.1007/BF00282757
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00282757