Abstract
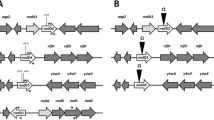
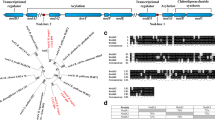
The two-component regulatory system Nod-VW of Bradyrhizobium japonicum is essential for the nodulation of the legume host plants Vigna radiata, V. unguiculata and Macroptilium atropurpureum. The NodV protein shares homology with the sensor-kinases, whereas the NodW protein is a member of the response-regulator class. We report here the identification of a new B. japonicum DNA region that is able to suppress the phenotypic defect of a nodW mutant, provided that this region is expressed from a foreign promoter. The minimal complementing region, which itself is not essential for nodulation in a nodW + background, consists of one gene designated nwsB (nodW-suppressor). The deduced amino acid sequence of the nwsB gene product shows a high degree of homology to NodW. The nwsB gene is preceded by a long open reading frame, nwsA, whose putative product appears to be a sensor-kinase. Downstream of nwsB, an open reading frame encoding a second putative response-regulator was identified. Interspecies hybridization revealed the presence of nwsAB-like DNA also in other Bradyrhizobium strains. Using nwsB′-′lacZ fusions, the nwsB gene was found to be expressed rather weakly in B. japonicum. This low level of expression is obviously not sufficient to compensate for a nodW − defect, whereas strong overexpression of nwsB is a condition that leads to suppression of the nodW − mutation.
Similar content being viewed by others
References
Albright LM, Huala E, Ausubel FM (1989) Prokaryotic signal transduction mediated by sensor and regulator protein pairs. Annu Rev Genet 23:311–336
Anthamatten D, Hennecke H (1991) The regulatory status of the fixL- and fixJ-like genes in Bradyrhizobium japonicum may be different from that in Rhizobium meliloti. Mol Gen Genet 225:38–48
Anthamatten D, Scherb B, Hennecke H (1992) Characterization of a fixLJ-regulated Bradyrhizobium japonicum gene sharing similarity with the Escherichia coli fnr and Rhizobium meliloti fixK genes. J Bacteriol 174:2111–2120
Beringer JE, Brewin NJ, Johnston AWB (1980) The genetic analysis of Rhizobium in relation to symbiotic nitrogen fixation. Heredity 45:161–186
Bott M, Bolliger M, Hennecke H (1990) Genetic analysis of the cytochrome c-aa 3 branch of the Bradyrhizobium japonicum respiratory chain. Mol Microbiol 4:2147–2157
Chiang RC, Cavicchioli R, Gunsalus RP (1992) Identification and characterization of narQ, a second nitrate sensor for nitrate-dependent gene regulation in Escherichia coli. Mol Microbiol 6:1913–1923
Corbin D, Barran L, Ditta G (1983) Organization and expression of Rhizobium meliloti nitrogen fixation genes. Proc Natl Acad Sci USA 80:3005–3009
Davis RW, Botstein D, Roth JR (1980) Advanced bacterial genetics. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, New York
Dénarié J, Debellé F, Rosenberg C (1992) Signaling and host range variation in nodulation. Annu Rev Microbiol 46:497–531
Dreyfus B, Garcia JL, Gillis M (1988) Characterization of Azorhizobium caulinodans gen. nov. sp. nov., a stem-nodulating nitrogen-fixing bacterium isolated from Sesbania rostrata. Int J Syst Bacteriol 38:89–98
Figurski DH, Helinski DR (1979) Replication of an origin-containing derivative of plasmid RK2 dependent on a plasmid function provided in trans. Proc Natl Acad Sci USA 76:1648–1652
Fisher RF, Long SR (1992) Rhizobium-plant signal exchange. Nature 357:655–660
Göttfert M (1993) Regulation and function of rhizobial nodulation genes. FEMS Microbiol Rev 104:39–64
Göttfert M, Lamb JW, Gasser R, Semenza J, Hennecke H (1989) Mutational analysis of the Bradyrhizobium japonicum common nod genes and further nod box-linked genomic DNA regions. Mol Gen Genet 215:407–415
Göttfert M, Grob P, Hennecke H (1990a) Proposed regulatory pathway encoded by the nodV and nodW genes, determinants of host specificity in Bradyrhizobium japonicum. Proc Natl Acad Sci USA 87:2680–2684
Göttfert M, Hitz S, Hennecke H (1990b) Identification of nodS and nodU, two inducible genes inserted between the Bradyrhizobium japonicum nodYABC and nodIJ genes. Mol Plant-Microbe Interact 3:308–316
Göttfert M, Holzhäuser D, Bäni D, Hennecke H (1992) Structural and functional analysis of two different nodD genes in Bradyrhizobium japonicum USDAl10. Mol Plant-Microbe Interact 5:257–265
Gross R, Arico B, Rappuoli R (1989) Families of bacterial signal-transducing proteins. Mol Microbiol 3:1661–1667
Györgypal Z, Kiss GB, Kondorosi A (1991) Transduction of plant signal molecules by the Rhizobium NodD proteins. Bioessays 13:575–581
Hahn M, Hennecke H (1984) Localized mutagenesis in Rhizobium japonicum. Mol Gen Genet 193:46–52
Hanahan D (1983) Studies of transformation of Escherichia coli with plasmids. J Mol Biol 166:557–563
Hennecke H, Günther I, Binder F (1982) A novel cloning vector for the direct selection of recombinant DNA in E. coli. Gene 19:231–234
Igo MM, Ninfa AJ, Stock JB, Silhavy TJ (1989) Phosphorylation and dephosphorylation of a bacterial transcriptional activator by a transmembrane receptor. Genes Dev 3:1725–1734
Kondorosi A, Kondorosi E, John M, Schmidt J, Schell J (1991) The role of nodulation genes in bacterium-plant communication. Genet Eng 13:115–136
Kullik I, Fritsche S, Knobel H, Sanjuan J, Hennecke H, Fischer H-M (1991) Bradyrhizobium japonicum has two differentially regulated, functional homologs of the σ54 gene (rpoN). J Bacteriol 173:1125–1138
Lamb JW, Hombrecher G, Johnston AWB (1982) Plasmid-determined nodulation and nitrogen fixation abilities in Rhizobium phaseoli. Mol Gen Genet 186:449–452
Lerouge P, Roche P, Faucher C, Maillet F, Truchet G, Promé JC, Dénarié J (1990) Symbiotic host-specificity of Rhizobium meliloti is determined by a sulphated and acylated glucosamine oligosaccharide signal. Nature 344:781–784
Long SR (1992) Genetic analysis of Rhizobium nodulation. In: Stacey G, Burris RH, Evans HJ (eds) Biological Nitrogen Fixation. Chapman and Hall, New York, pp 560–597
Matsumura P, Rydel JJ, Linzmeier R, Vacante D (1984) Overexpression and sequence of the Escherichia coli cheY gene and biochemical activities of the CheY protein. J Bacteriol 160:36–41
Messing J (1983) New M13 vectors for cloning. Methods Enzymol 101:20–78
Miller JH (1972) Experiments in molecular genetics. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, New York
Mutoh N, Simon MI (1986) Nucleotide sequence corresponding to five chemotaxis genes in Escherichia coli. J Bacteriol 165:161–166
Nagasawa S, Tokishita S, Aiba H, Mizuno T (1992) A novel sensor-regulator protein that belongs to the homologous family of signal-transduction proteins involved in adaptive responses in Escherichia coli. Mol Microbiol 6:799–807
Ninfa AJ (1991) Protein phosphorylation and the regulation of cellular processes by the homologous two-component regulatory systems of bacteria. Genet Eng 13:39–72
Ninfa AJ, Ninfa EG, Lupas AN, Stock A, Magasanik B, Stock J (1988) Crosstalk between bacterial chemotaxis signal transduction proteins and regulators of transcription of the Ntr regulon evidence that nitrogen assimilation and chemotaxis are controlled by a common phosphotransfer mechanism. Proc Natl Acad USA 85:5492–5496
Parkinson JS, Kofoid EC (1992) Communication modules in bacterial signaling proteins. Annu Rev Genet 26:71–112
Price NPJ, Relic B, Talmont E, Lewin A, Promé D, Pueppke SG, Maillet F, Dénarié J, Promé JC, Broughton WJ (1992) Broad-host-range Rhizobium species strain NGR234 secretes a family of carbamoylated, and fucosylated, nodulation signals that are O-acetylated or sulphated. Mol Microbiol 6:3575–3584
Rabin RS, Stewart V (1992) Either of two functionally redundant sensor proteins, NarX and NarQ, is sufficient for nitrate regulation in Escherichia coli K-12. Proc Natl Acad Sci USA 89:8419–8423
Ramseier TM, Winteler HV, Hennecke H (1991) Discovery and sequence analysis of bacterial genes involved in the biogenesis of c-type cytochromes. J Biol Chem 266:7793–7803
Regensburger B, Hennecke H (1983) RNA polymerase from Rhizobium japonicum. Arch Microbiol 135:103–109
Rostas K, Kondorosi E, Horvath B, Simoncsits A, Kondorosi A (1986) Conservation of extended promoter regions of nodulation genes in Rhizobium. Proc Natl Acad Sci USA 83:1757–1761
Sambrook J, Fritsch EF, Maniatis T (1989) Molecular cloning: A laboratory manual, 2nd edn. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, New York
Sanger F, Nicklen S, Coulson AR (1977) DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci USA 74:5463–5467
Sanjuan J, Carlson RW, Spaink HP, Bhat UR, Barbour WM, Glushka J, Stacey G (1992) A 2-O-methyl-fucose moiety is present in the lipo-oligosaccharide nodulation signal of Bradyrhizobium japonicum. Proc Natl Acad Sci USA 89:8789–8793
Schlaman HRM, Okker RJH, Lugtenberg BJ (1992) Regulation of nodulation gene expression by NodD in rhizobia. J Bacteriol 174:5177–5182
Schultze M, Quiclet-Sire B, Kondorosi E, Virelizier H, Glushka JN, Endre G, Géro SD, Kondorosi A (1992) Rhizobium meliloti produces a family of sulfated lipo-oligosaccharides exhibiting different degrees of plant host specificity. Proc Natl Acad Sci USA 89:192–196
Simon R, Priefer U, Pühler A (1983) Vector plasmids for in vivo and in vitro manipulation of gram-negative bacteria. In: Pühler A (ed) Molecular genetics of the bacteria-plant interaction. Springer Verlag, Heidelberg, pp 98–106
Simon R, Quandt J, Klipp W (1989) New derivatives of transposon Tn5 suitable for mobilization of replicons, generation of operon fusions and induction of genes in Gram-negative bacteria. Gene 80:161–169
Spaink HP (1992) Rhizobial lipo-oligosaccharides: answers and questions. Plant Mol Biol 20:977–986
Spaink HP, Sheeley DM, van Brussel AAN, Glushka J, York WS, Tak T, Geiger O, Kennedy EP, Reinhold VN, Lugtenberg BJJ (1991) A novel highly unsaturated fatty acid moiety of lipo-oligosaccharide signals determines host specificity of Rhizobium. Nature 354:125–130
Spaink HP, Aarts A, Stacey G, Bloemberg GV, Lugtenberg BJJ, Kennedy EP (1992) Detection and separation of Rhizobium and Bradyrhizobium Nod metabolites using thin-layer chromatography. Mol Plant-Microbe Interact 5:72–80
Stock JB, Ninfa AJ, Stock AM (1989) Protein phosphorylation and regulation of adaptive responses in bacteria. Microbiol Rev 53:450–490
Trach KA, Chapman JW, Piggot PJ, Hoch JA (1985) Deduced product of the stage 0 sporulation gene spoOF shares homology with the SpoOA, OmpR, and SfrA proteins. Proc Natl Acad Sci USA 82:7260–7264
Wanner BL (1992) Is cross regulation by phosphorylation of twocomponent response regulator proteins important in bacteria? J Bacteriol 174:2053–2058
Author information
Authors and Affiliations
Additional information
Communicated by A. Kondorosi
Rights and permissions
About this article
Cite this article
Grob, P., Michel, P., Hennecke, H. et al. A novel response-regulator is able to suppress the nodulation defect of a Bradyrhizobium japonicum nodW mutant. Molec. Gen. Genet. 241, 531–541 (1993). https://doi.org/10.1007/BF00279895
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00279895