Summary
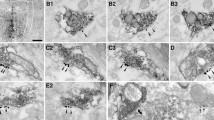
To determine if PAG stimulation activates serotonin containing neurons in RM, intracellular techniques were used to identify raphe magnus (RM) cells that are excited by antinociceptive periaqueductal gray (PAG) stimulation in the cat. RM neurons that received a monosynaptic EPSP from PAG shock were intracellularly labeled with ethidium bromide. Intracellularly stained RM cells were large, medium and small sized neurons. To determine if these cells contain serotonin, sections containing intracellularly labeled RM neurons were processed for serotonin immunocytochemistry. None of the RM cells (N = 32) which were intracellularly stained with ethidium bromide contained immunoreactive serotonin. These results are evidence that non-serotonergic RM cells are activated by antinociceptive PAG stimulation; these cells may be important in the relay of PAG induced antinociception.
Similar content being viewed by others
References
Ackerman E, Chrubaskik J, Weinstock M, Wunsch E (1985) Effect of intrathecal somatostatin on pain threshold in rats. Schmerz 2: 41–42
Aghajanian GK, Vandermaelen CP (1982) Intracellular identification of central noradrenergic and serotonergic neurons by a new double labeling procedure. J Neurosci 2: 1786–1792
Anderson EG, Proudfit HK (1981) The functional role of the bulbospinal serotonergic nervous system. In: Jacobs BL, Gelperin A (eds) Serotonergic neurotransmission and behavior. MIT Press, Cambridge, pp 307–338
Barbaro NM, Hammond DL, Fields HL (1985) Effects of intrathecally administered methysergide and yohimbine on microstimulation-produced antinociception in the rat. Brain Res 343: 223–229
Basbaum AI, Fields HL (1979) The origin of descending pathways in the dorsolateral funiculus of the spinal cord of the cat and rat: further studies on the anatomy of pain modulation. J Comp Neurol 187: 513–531
Basbaum AI, Clanton CH, Fields HL (1978) Three bulbospinal pathways from the rostral medulla of the cat: an autoradiographic study of pain modulating systems. J Comp Neurol 178: 209–224
Behbehani MM, Fields HL (1979) Evidence that an excitatory connection between the periaqueductal gray and nucleus raphe magnus mediates stimulation produced analgesia. Brain Res 170: 85–93
Belin MF, Nanopoulos D, Didier M, Aguera M, Steinbusch H, Verhofstad A, Maitre M, Pujol JF (1983) Immunohistochemical evidence in one nerve cell. A study on the raphe nuclei of the rat using antibodies to glutamate decarboxylase and serotonin. Brain Res 275: 329–339
Berman AL (1968) The brainstem of the cat. University of Wisconsin Press, Madison
Bowker RM, Westlund KN, Sullivan MC, Wilber JF, Coulter JD (1983) Descending serotonergic, peptidergic and cholinergic pathways from the raphe nuclei: a multiple transmitter complex. Brain Res 288: 33–48
Dahlström A, Fuxe K (1965) Evidence for the existence of monoamine neurons in the central nervous system. II. Experimentally induced changes in the intraneuronal amine levels of bulbospinal neuron systems. Acta Physiol Scand [Suppl 247] 64: 1–36
Doi T, Jurna I (1981) Intrathecal substance P depresses the tail flick response — antagonism by naloxone. Arch Pharmacol 317: 135–139
Finley JCW, Maderdrut JL, Roger LJ, Petrusz P (1981) The immunocytochemical localization of somatostatin-containing neurons in the rat central nervous system. Neuroscience 6: 2173–2192
Fox GQ, Pappas GD, Purpura DP (1976) Morphology and fine structure of the feline neonatal medullary raphe nuclei. Brain Res 101: 385–410
Giesler GJ, Gerhart KD, Yezierski RP, Wilcox TK, Willis WD (1981) Postsynaptic inhibition of primate spinothalamic neurons by stimulation in nucleus raphe magnus. Brain Res 204: 184–188
Hammond DL (1985) Pharmacology of central pain-modulating networks (biogenic amines and nonopioid analgesics). Adv Pain Res Ther 9: 499–511
Hammond DL, Yaksh TL (1984) Antagonism of stimulationproduced antinociception by intrathecal administration of methysergide or phentolamine. Brain Res 298: 329–337
Hammond DL, Levy RA, Proudfit HL (1980) Hypoalgesia induced by microinjection of a norepinephrine antagonist in the nucleus raphe magnus: reversal by intrathecal administration of a serotonin antagonist. Brain Res 201: 475–479
Hökfelt T, Ljungdahl A, Steinbusch H, Verhofstad A, Nilsson G, Brodin E, Pernow B, Goldstein M (1978) Immunohistochemical evidence of substance P-like immunoreactivity in some 5-hydroxytryptamine-containing neurons in the rat central nervous system. Neuroscience 3: 517–538
Holstege G, Kuypers HGJM (1982) The anatomy of brainstem pathways to the spinal cord in cat. A labeled amino acid tracing study. Prog Brain Res 57: 145–175
Hylden JLK, Hayashi H, Ruda MA, Dubner R (1986) Serotonin innervation of physiologically identified lamina I projection neurons. Brain Res 370: 401–404
Jensen T, Yaksh TL (1985) Spinal monoamine and opiate systems partly mediate the antinociceptive effects of glutamate microinjected at brain stem sites. Brain Res 321: 287–297
Johansson O, Hökfelt T, Pernow B, Jeffcoate SL, White N, Steinbusch HWM, Verhofstad AAJ, Emson PC, Spindel E (1981) Immunohistochemical support for three putative transmitters in one neuron: coexistence of 5-hydroxytryptamine, substance P- and thyrotropin releasing hormone-like immunoreactivity in medullary neurons projecting to the spinal cord. Neuroscience 6: 1857–1881
Lederer WJ, Spindler AJ, Eisner DA (1979) Thick slurry bevelling: a new technique for bevelling extremely fine microelectrodes and micropipettes. Pflügers Arch 381: 287–288
Leger L, Charnay Y, Dubois PM, Jouvet M (1986) Distribution of enkephalin-immunoreactive cell bodies in relation to serotonin-containing neurons in the raphe nuclei of the cat: immunohistochemical evidence for the coexistence of enkephalins and serotonin in certain cells. Brain Res 362: 63–73
Light AR, Casale EJ, Menetrey DM (1986) The effects of focal stimulation in nucleus raphe magnus and periaqueductal gray on intracellularly recorded neurons in spinal laminae I and II. J Neurophysiol 56: 555–571
Lovick TA, Hunt SP (1983) Substance P-immunoreactive and serotonin-containing neurones in the ventral brainstem of the cat. Neurosci Lett 36: 223–228
Maciewicz R, Sandrew BB, Phipps BS, Poletti CE, Foote WE (1983) Pontomedullary raphe neurons: intracellular responses to central and peripheral electrical stimulation. Brain Res 293: 17–33
Mantyh PW, Hunt SP (1984) Evidence for cholecystokinin-like immunoreactive neurons in the rat medulla oblongata which project to the spinal cord. Brain Res 291: 49–54
Mason P, Strassman A, Maciewicz R (1985) Pontomedullary raphe neurons: monosynaptic excitation from midbrain sites that suppress the jaw opening reflex. Brain Res 329: 384–389
Mason P, Strassman A, Maciewicz R (1986) Intracellular responses of raphe magnus neurons during the jaw-opening reflex evoked by tooth pulp stimulation. Brain Res 379: 232–241
Mason P, Strassman A, Maciewicz R (1986b) Immunocytochemistry of electrophysiologically characterized raphe magnus neurons. Abstract, Society for Neuroscience
Mayer DJ, Wolfe TL, Akil H, Carder B, Liebeskind JC (1971) Analgesia from electrical stimulation in the brainstem of the rat. Science 174: 1351–1354
Messing RB, Lytle LD (1977) Serotonin-containing neurons: their possible role in pain and analgesia. Pain 4: 1–21
Oliveras JL, Bourgoin S, Hery F, Besson JM, Hamon M (1977) The topographical distribution of serotonergic terminals in the spinal cord of the cat: biochemical mapping by the combined use of microdissection and microassay procedures. Brain Res 138: 393–406
Oliveras JL, Guilbaud G, Besson JM (1979) A map of serotoninergic structure involved in stimulation producing analgesia in unrestrained freely moving cats. Brain Res 164: 317–322
Pittaway KM, Rodriguez RE, Hughes J, Hill RG (1987) CCK 8 analgesia and hyperalgesia after intrathecal administration in the rat: comparison with CCK-related peptides. Neuropeptides 10: 87–108
Rivot JP, Chaouch A, Besson JM (1980) Nucleus raphe magnus modulation of response of rat dorsal horn neurons to unmyelinated fiber inputs: partial involvement of serotonergic pathways. J Neurophysiol 44: 1039–1057
Skagerberg G, Björklund A (1985) Topographic principles in the spinal projections of serotonergic and non-serotonergic brainstem neurons in the rat. Neuroscience 15: 445–480
Wessendorf MW, Proudfit HK, Anderson EG (1981) The identification of serotonergic neurons in the nucleus raphe magnus by conduction velocity. Brain Res 214: 168–173
Yaksh TL (1983) In vivo studies on antinociceptive in the spinal intrathecal space of animals. Eur J Pharmacol 51: 323–330
Yezierski RP, Wilcox TK, Willis WD (1982) The effects of serotonin antagonists on the inhibition of primate spinothalamic tract cells produced by stimulation in nucleus raphe magnus or periaqueductal gray. J Pharmacol Exp Ther 220: 266–277
Zahs K, Lakos S, Basbaum AI (1985) Immunoreactive serotonergic axons in the dorsolateral funiculus (DLF) of the cat and rat. Soc Neurosci Abstr 11: 125
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Mason, P., Strassman, A. & Maciewicz, R. Serotonin immunocytochemistry of physiologically characterized raphe magnus neurons. Exp Brain Res 73, 1–7 (1988). https://doi.org/10.1007/BF00279654
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00279654