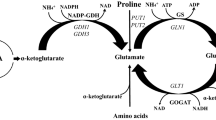
Summary
In Saccharomyces cerevisiae, the products of eleven different genes are needed for a functional sulfate assimilation pathway. Only five enzymatic steps are known in this pathway. The study of the gene-enzyme relationships has shown that the enzymes catalysing two of these steps are probably heteropolymeric. Moreover, mutations in three unlinked genes lead to multiple enzymatic losses. Different hypotheses are made to account for these results.
Similar content being viewed by others
References
Antoniewski, J.: Regulation de la voie de biosynthèse de la méthionine chez Saccharomyces cerevisiae: aspects physiologiques. Thèse de Doctorat-ingénieur. Université de Paris-Sud, 1972
Antoniewski, J., Robichon-Szulmajster, H. de: Biosynthesis of methionine and its control in wild type and regulatory mutants of Saccharomyces cerevisiae. Biochimie 55, 529–539 (1973)
Breton, A., Surdin-Kerjan, Y.: Sulfate uptake in Saccharomyces cerevisiae: Biochemical and genetic study. Submitted to J. Bact. (1977)
Cherest, H., Eichler, F., Robichon-Szulmajster, H. de: Genetic and regulatory aspects of methionine biosynthesis in Saccharomyces cerevisiae. J. Bact. 97, 328–336 (1969)
Cherest, H., Surdin-Kerjan, Y., Robichon-Szulmajster, H. de: Methionine mediated repression in Saccharomyces cerevisiae. A pleiotropic regulatory system involving methionyl transfer ribonucleic acid and the product of gene ETH2. J. Bact. 106, 758–772 (1971)
Dreyfuss, J., Monty, K.J.: The biochemical characterization of cystein requiring mutants of Salmonella typhimurium. J. biol. Chem. 328, 1019–1024 (1963)
Fink, G.R.: Gene clusters and the regulation of biosynthetic pathway in fungi. In: Metabolic pathways. V. Metabolic regulation (ed. H.J. Vogel), pp. 199–223. New York: Academic Press 1971
Giles, N.H., Case, M.E., Dartridge, C.W.H., Ahmed, S.I.: A gene cluster in Neurospora crassa coding for an aggregate of five aromatic synthetic enzymes. Proc. nat. Acad. Sci. (Wash.) 58, 1453–1460 (1967)
Grant, W.M.: Colorimetric determination of sulfur dioxyde. Anal. Chem. 9, 345–346 (1947)
Jacob, F., Monod, J.: On the regulation of gene activity. Cold Spr. Harb. Symp. quant. Biol. 26, 193–211 (1961)
Knobling, A., Schiffmann, D., Sickinger, H.D., Schweizer, E.: Malonyl and palmityl transferase less mutants of the yeast fatty acid synthetase complex. Europ. J. Biochem. 56, 359–367 (1975)
Kredich, N.M., Tomkins, G.N.: The enzyme synthesis of L-cysteine in E. coli and S. typhimurium. J. biol. Chem. 241, 4955–4965 (1966)
Masselot, M., Robichon-Szulmajster, H. de: Nonsense mutation in the regulatory gene ETH2 involved in methionine biosynthesis in Saccharomyces cerevisiae. Genetics 71, 535–550 (19 )
Masselot, M., Robichon-Szulmajster, H. de: Methionine biosynthesis in Saccharomyces cerevisiae. I. Genetical analysis of auxotrophic mutants. Molec. gen. Genet. 139, 121–132 (1975)
Mortimer, R.K., Hawthorne, D.C.: Genetic mapping in Saccharomyces cerevisiae. IV. Mapping of temperature sensitive genes and use of disomic strains in localizing genes. Genetics 74, 33–54 (1973)
Moss, J.A. de: Biochemical diversity in the tryptophan pathway. Biochem. biophys. Res. Commun. 18, 850–857 (1965)
Moss, J.A. de, Weigman, J.: An enzyme aggregate in the tryptophan pathway of Neurospora crassa. Proc. nat. Acad. Sci. (Wash.) 54, 241–247 (1965)
Naiki, N.: Enzymatic defects in sulfate reducing system of sulfiteless yeast mutants. Plant and Cell Physiol. 5, 71–78 (1964)
Naiki, N.: Some properties of sulfite reductase in yeast. Plant and Cell Physiol. 6, 179–194 (1965)
Pasternak, C.A., Ellis, R.J., Jones-Mortimer, M.C., Crichton, C.E.: The control of sulphate reduction in bacteria. Biochem. J. 96, 270–275 (1965)
Robbins, P.W.: Sulfate activating enzymes. In: Methods in enzymology (ed. S.P. Colowick and N.O- Kaplan), vol. V, pp. 964–977. New York: Academic Press 1962
Robbins, P.W.: Preparation and properties of sulfuryl adenylates. In: Methods in enzymology (ed. S.P. Colowick and N.O. Kaplan), Vol. VI, pp. 766–775. New York: Academic Press 1963
Robbins, P.W., Lipmann, F.: Enzymatic synthesis of adenosine-5′-phosphosulfate. J. biol. Chem. 233, 685–690 (1958)
Siegel, L.M.: A direct microdetermination for sulfide. Anal. Biochem. 11, 126–132 (1965)
Spence, K.D.: Mutation of Saccharomyces cerevisiae preventing uptake of S-adenosyl methionine. J. Bact. 106, 325–330 (1971)
Vito, P.C. de, Dreyfuss, J.: Metabolic regulation of adenosine triphosphate sulfurylase in yeast. J. Bact. 88, 1341–1348 (1964)
Wainwright, T.: Reduction of sulfite by yeast enzymes. Biochem. J. 83, 39P (1962)
Wiebers, J.L., Garner, H.R.: Acyl derivatives of homoserine as substrates for homocysteine synthesis in N. crassa, yeast and E. coli. J. biol. Chem. 242, 5644–5649 (1967)
Wilson, L.G., Bandurski, R.S.: Enzymatic reactions involving sulfate, sulfite, selenate and molybdate. J. biol. Chem. 233, 975–981 (1958)
Yamagata, S., Takeshima, K., Naiki, N.: Evidence for the identity of O-acetylserine sulfhydrylase with O-acetylhomoserine sulfhydrylase in Yeast. J. Biochem. 75, 1221–1229
Yamagata, S., Takeshima, K., Naiki, N.: O-acetylserine and O-acetylhomoserine sulfhydrylase of yeast; studies with methionine auxotrophs. J. Biochem. 77, 1029–1036 (1975)
Yoshimoto, A., Sato, R.: Studies on yeast sulfite reductase. I. Purification and characterization. Biochim. biophys. Acta (Amst.) 153, 555–575 (1968a)
Yoshimoto, A., Sato, R.: Studies on yeast sulfite reductase. II. Partial purification and properties of genetically incomplete sulfite reductase. Biochim. biophys. Acta (Amst.) 153, 576–588 (1968b)
Author information
Authors and Affiliations
Additional information
Communicated by F. Kaudewitz
Rights and permissions
About this article
Cite this article
Masselot, M., Surdin-Kerjan, Y. Methionine biosynthesis in Saccharomyces cerevisiae . Molec. Gen. Genet. 154, 23–30 (1977). https://doi.org/10.1007/BF00265572
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF00265572