Summary
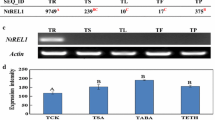
A family of cross-hybridising cDNA clones has been isolated from a cDNA library produced with poly(A)+ RNA from the roots of oilseed-rape (Brassica napus L.). The clones were selected as abundantly expressed in root by differential screening of the root cDNA library with cDNA probes prepared from root, green leaf, etiolated leaf and developing seed. mRNA species corresponding to the selected abundant clones were expressed in roots at levels of at least 400 times those in other organs, as shown by Northern blot analysis and RNase protection assays. Complete nucleotide sequence determination of the cDNA clones showed that they encoded proteins homologous to carrot extensin and were the products of at least three different genes. An extensin gene, designated extA, was obtained from an oilseed rape (B. napus L.) genomic library screened with a cDNA species encoding a protein expressed abundantly in roots. The gene is a member of a multigene family, consisting of about 3 members per haploid genome with strong homology to the probe, and a further 20 or so members with weaker homology. The isolated gene, although not identical to the cDNA probe, was also found to be specifically expressed in roots, and was transcribed into a mRNA species approximately 1300 nucleotides in size. A single transcription start was identified by S1 mapping. The complete nucleotide sequence of the extA gene and its flanking regions has been determined and shown to encode a protein homologous to carrot and tomato extensins.
Similar content being viewed by others
References
Albretsen C, Haukanes B-I, Aasland R, Kleppe K (1988) Optimal conditions for hybridisation with oligonucleotides: A study with myc-oncogene DNA probes. Anal Biochem 170:193–202
Aviv H, Leder P (1972) Purification of biologically active globin messenger RNA by chromatography on oligothymidylic acidcellulose. Proc Natl Acad Sci USA 69:1408–1412
Bennet MD, Smith DB (1976) Nuclear DNA amounts in angiosperms. Philos Trans R Soc Lond [Biol] 274:227–273
Cassab GI, Varner JE (1987) Immunocytolocalization of extensin in developing soybean seed coats by immunogold-silver staining and by tissue printing on nitrocellulose paper. J Cell Biol 105:2581–2588
Cassab GI, Varner JE (1988) Cell wall proteins. Annu Rev Plant Physiol Plant Mol Biol 39:321–353
Chen JA, Varner JE (1985a) Isolation and characterization of cDNA clones for carrot extensin and a proline-rich 33-kDa protein. Proc Natl Acad Sci USA 82:4399–4403
Chen JA, Varner JE (1985b) An extracellular matrix protein in plants: characterization of a genomic clone for carrot extensin. EMBO J 4:2145–2151
Chirgwin JM, Przybyla AE, Macdonald RJ, Rutter WJ (1979) Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry 8:5294–5299
Chrispeels MJ, Sadava D, Cho YP (1974) Enhancement of extensin biosynthesis in aging discs of carrot storage tissue. J Exp Bol 25:1157–1166
Cooper JB (1988) Cell wall extensin genes. In: Verma DPS, Goldberg RB (eds) Plant Gene Research, vol. 5, Temporal and Spatial Regulation of Plant Genes. Springer-Verlag, Wien, New York, pp 235–249
Croy RRD, Lycett GW, Gatehouse JA, Yarwood JN, Boulter D (1982) Cloning and analysis of cDNAs encoding plant storage protein precursors. Nature 295:76–79
Dietrich RA, Maslyar DJ, Hempel RC, Harada JJ (1989) Spatial patterns of gene expression in Brassica napus seedlings: identification of a cortex-specific gene and localization of mRNAs encoding isocitrate lyase and a polypeptide homologous to proteinases. Plant Cell 1:73–80
Evans IM, Croy RRD, Brown P, Boulter D (1980) Synthesis of complementary DNAs to partially purified mRNAs coding for the storage proteins of Pisum sativum L. Biochem Biophys Acta 610:81–95
Evans IM, Swinhoe R, Gatehouse LN, Gatehouse JA, Boulter D (1988) Distribution of root mRNA species in other vegetative organs of pea (Pisum sativum L.). Mol Gen Genet 214:153–157
Favaloro J, Treisman R, Kamen R (1980) Transcription maps of polyoma virus-specific RNA: analysis by two-dimensional nuclease S1 mapping. Methods Enzymol 65:718–749
Feinberg AP, Vogelstein B (1983) A technique for radiolabeling DNA restriction endonuclease fragments to high-specific activity. Anal Biochem 132:6–13
Frischauf AM, Lehrach H, Poustka A, Murray N (1983) Lambda replacement vectors carrying polylinker sequences. J Mol Biol 170:827–842
Gatehouse JA, Evans IM, Bown D, Croy RRD, Boulter D (1982) Control of storage-protein synthesis during seed development in pea (Pisum sativum L.). Biochem J 250:15–24
Glišin V, Crkvenjakov R, Byus C (1974) Ribonucleic acid isolated by cesium chloride centrifugation. Biochemistry 13:2633–2637
Goldberg RB, Barker SJ, Perez-Grau L (1989) Regulation of gene expression during plant embryogenesis. Cell 56:149–160
Graham DE (1978) The isolation of high molecular weight DNA from whole organisms or large tissue masses. Anal Biochem 85:609–613
Hall TC, Ma Y, Buchbinder BU, Pyne JW, Sun SM, Bliss FA (1978) Messenger RNA for G1 protein of French bean seeds: cell-free translation and product characterisation. Proc Natl Acad Sci USA 75:3196–3200
Henikoff S (1984) Unidirectional digestion with exonuclease III creates targeted breakpoints for DNA sequencing. Gene 28:351–359
Holdsworth MJ, Laties GG (1989) Site-specific binding of a nuclear factor to the carrot extensin gene is influenced by both ethylene and wounding. Planta 179:17–23
Hopp TP, Wood KR (1981) Prediction and protein antigenic determinants from amino acid sequences. Proc Natl Acad Sci USA 78:3824–3828
Jones RL, Robinson DG (1989) Protein secretion in plants. New Phytol 111:567–597
Krieg PA, Melton DA (1987) In vitro RNA synthesis with SP6 RNA polymerase. Methods Enzymol 155:397–415
Kiihn S, Fritz H-J, Starlinger P (1979) Close vicinity of IS 1 integration sites in the leader sequence of the gal operon of E. coli. Mol Gen Genet 167:235–241
Lamport DTA (1965) The protein component of primary cell walls. Adv Bot Res 2:151–218
Lamport DTA (1977) Structure, biosynthesis and significance of cell wall glycoproteins. In: Loewus FA, Runeckles VC (eds) Recent Advances in Phytochemistry, vol 2. Plenum Press, New York, pp 79–115
Lamport DTA, Northcote DA (1960) Hydroxyproline in primary cell walls of higher plants. Nature 118:665–666
Lycett GW, Croy RRD, Shirsat AH, Boulter D (1984) The complete nucleotide sequence of a legumin gene from pea (Pisum sativum L.). Nucleic Acids Res 12:4493–4506
Maniatis T, Fritsch EF, Sambrook J (1982) Molecular cloning: a laboratory manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, New York
Marzluff WF, Huang RCC (1984) Transcription of RNA in isolated nuclei. In: Hames BD, Higgins SJ (eds) Transcription and Translation. IRL Press, Oxford, pp 89–129
Maxam AM, Gilbert W (1980) Sequencing end-labelled DNA with base-specific chemical cleavages. Methods Enzymol 65:499–560
McMaster GK, Carmichael GG (1977) Analysis of single and double stranded nucleic acids on polyacrylamide and agarose gels by using glyoxal and acridine orange. Proc Natl Acad Sci USA 74:4835–4838
Melton DA, Krieg PA, Rebagliali MR, Maniatis T, Zinn K, Green MR (1984) Efficient in vitro synthesis of biologically active RNA and RNA hybridisation probes from plasmids containing a bacteriophage SP6 promoter. Nucleic Acids Res 12:7035–7056
Messing J (1983) New M13 vectors for cloning. Methods Enzymol 101:20–78
Messing J, Geraghty D, Heidecker G, Hu N-T, Kridl J, Rubinstein I (1983) Plant gene structure. In: Kosuge T, Meredith CP, Hollaender A (eds) Genetic Engineering of Plants. Plenum Publishing Corporation, New York, pp 211–227
Prat S, Cortadas J, Puigdomenech P, Palau J (1985) Nucleic acid (cDNA) and amino acid sequences of the maize endosperm protein glutelin-2. Nucleic Acids Res 13:1493–1504
Rosahl S, Eckes P, Schell J, Willmitzer L (1986) Organ-specific gene expression in potato: isolation and characterisation of tuber-specific cDNA sequences. Mol Gen Genet 202:368–373
Sanger F, Nicklen S, Coulson AR (1977) Sequencing with chainterminating inhibitors. Proc Natl Acad Sci USA 74:5463–5467
Showalter AM, Varner JE (1987) Molecular details of plant cell wall hydroxyproline-rich glycoprotein expression during wounding and infection. UCLA Symp Mol Cell Biol 48:375–392
Smith JJ, Muldoon EP, Willard JJ, Lamport DTA (1986) Tomato extensin precursors P1 and P2 are highly periodic structures. Phytochemistry 25:1021–1030
Southern EM (1979) Gel electrophoresis of restriction fragments. Methods Enzymol 68:152–176
Thomas PS (1980) Hybridisation of denatured RNA and small DNA fragments transferred to nitrocellulose. Proc Natl Acad Sci USA 77:5201–5205
Thompson AJ, Evans IM, Boulter D, Croy RRD, Gatehouse JA (1989) Transcriptional and post-transcriptional regulation of seed storage-protein gene expression in pea (Pisum sativum L.). Planta 179:279–287
von Heijne G (1985) Signal sequences. The limits of variation. J Mol Biol 184:99–105
Walling L, Drews GN, Goldberg RB (1986) Transcriptional and post-transcriptional regulation of soybean seed protein mRNA levels. Proc Natl Acad Sci USA 83:2123–2127
Wickens MP, Buell GN, Schimke RT (1978) Synthesis of doublestranded DNA complementary to lysozyme, ovomucoid and ovalbumin mRNAs. J Biol Chem 253:2483–2495
Author information
Authors and Affiliations
Additional information
Communicated by J. Schell
Rights and permissions
About this article
Cite this article
Marta Evans, I., Gatehouse, L.N., Gatehouse, J.A. et al. The extensin gene family in oilseed rape (Brassica napus L.): Characterisation of sequences of representative members of the family. Molec. Gen. Genet. 223, 273–287 (1990). https://doi.org/10.1007/BF00265064
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF00265064