Summary
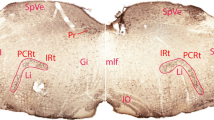
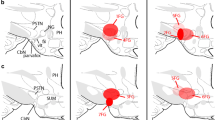
In order to elucidate the geometric organization of projections from the barrel cortex to the thalamus, iontophoretic injections of the anterograde tracer Phaseolus vulgaris-leucoagglutinin were made. The injections were confined to one barrel column (i.e. barrel in layer IV + cortical tissue above and below it). Axonal terminations could be demonstrated in three thalamic nuclei: reticularis (RT), ventrobasalis (VB) and posterior (PO). Anterograde terminal labelling was obtained in RT + VB; in PO only; or in RT + VB + PO. The terminals labelled in PO were much larger than those in RT and VB. The termination areas in RT, VB and PO were shaped like rods which have a rostro-caudal orientation. These cortico-thalamic projections are discretely and topographically organized. The clearest such arrangement was found in VB. Here, projections from the A row of barrels in BF terminate dorsally, whereas those from the C row end ventrally. Barrel A1 projects to the lateral part of VB, whereas A4, to more medial parts; other rows are arranged similarly. These results were compared with the distribution of thalamo-cortical projection neurons that were labelled after iontophoretic HRP injections in individual barrels. We concluded that the corticothalamic projections originating from one barrel column contact an arc of barreloids in VB.
Similar content being viewed by others
References
Akers RM, Killackey HP (1979) Segregation of cortical and trigeminal afferents to the ventrobasal complex of the neonatal rat. Brain Res 161: 527–532
Bates CA, Killackey HP (1985) The organization of the neonatal rat's brainstem trigeminal complex and its role in the formation of central trigeminal patterns. J Comp Neurol 240: 265–287
Belford GR, Killackey HP (1979) Vibrissae representation in subcortical trigeminal centers of the neonatal rat. J Comp Neurol 183: 305–322
Cajal S Ramón y (1911) Histologie du système nerveux de l'homme et des vertébrés. Maloine, Paris
Cipolloni PB, Hersch SM, White EL (1985) The use of lectin transport in the mouse central nervous system as an anterograde axonal marker for electron microscopy. Neurosci Lett 58: 43–47
Crandall JE, Caviness VS Jr (1984) Thalamocortical connections in newborn mice. J Comp Neurol 228: 542–556
Davidson N (1965) The projection of afferent pathways on the thalamus of the rat. J Comp Neurol 124: 377–390
Donaldson L, Hand PJ, Morrison AR (1975) Cortical-thalamic relationships in the rat. Exp Neurol 47: 448–458
Donoghue JP, Kerman KL, Ebner FF (1979) Evidence for two organizational plans within the somatic sensory-motor cortex of the rat. J Comp Neurol 183: 647–664
Durham D, Woolsey TA (1984) Effects of neonatal whisker lesions on mouse central trigeminal pathways. J Comp Neurol 223: 424–447
Erzurumlu RS, Killackey HP (1980) Diencephalic projections of the subnucleus interpolaris of the brainstem trigeminal complex in the rat. Neurosci 5: 1891–1901
Frost DO, Caviness VS Jr (1980) Radial organization of thalamic projections to the neocortex in the mouse. J Comp Neurol 194: 369–393
Fukushima T, Kerr FWL (1979) Organization of trigeminothalamic tracts and other thalamic afferent systems of the brainstem in the rat: presence of gelatinosa neurons with thalamic connections. J Comp Neurol 183: 169–184
Gerfen CR, Sawchenko PE (1984) An anterograde neuroanatomical tracing method that shows the detailed morphology of neurons, their axons and terminals: immunohistochemical localization of an axonally transported plant lectin, Phaseolus vulgris-leucoagglutinin (PHA-L). Brain Res 290: 219–238
Gonzalez MF, Sharp FR (1985) Vibrissae tactile stimulation: (14C)2-deoxyglucose uptake in rat brainstem, thalamus, and cortex. J Comp Neurol 231: 457–472
Hanker JS, Yates PE, Metz CB, Rustioni A (1977) A new specific, sensitive and non-carcinogenic reagent for the demonstration of horseradish peroxidase. J Histochem 9: 789–792
Jeanmonod D, Rice FL, Van der Loos H (1981) Mouse somatosensory cortex: alterations in the barrelfield following receptor injury at different early postnatal ages. Neuroscience 6: 1503–1535
Jones EG (1975) Some aspects of the organization of the thalamic reticular complex. J Comp Neurol 162: 285–308
Jones EG (1986) The thalamus. Plenum Press, New York, London
Keller A, White EL, Cipolloni PB (1985) The identification of thalamocortical axon terminals in barrels of mouse SmI cortex using immunohistochemistry of anterograde transported leetin (Phaseolus vulgaris-leucoagglutinin). Brain Res 343: 159–165
Killackey HP, Belford GR (1979) The formation of afferent patterns in the somatosensory cortex of the neonatal rat. J Comp Neurol 183: 285–304
Killackey HP, Fleming K (1985) The role of the principle sensory nucleus in central trigeminal pattern formation. Dev Brain Res 22: 141–145
Landry P, Dykes RW (1985) Identification of two populations of corticothalamic neurons in cat primary somatosensory cortex. Exp Brain Res 60: 289–298
McAllister JP, Wells J (1981) The structural organization of the ventral posterolateral nucleus in the rat. J Comp Neurol 197: 271–301
Melzer P, Van der Loos H, Dörfl J, Welker E (1985) A magnetic device to stimulate selected whiskers of freely moving or restrained small rodents: its application in a deoxyglocuse study. Brain Res 348: 229–240
Nussbaumer J-C, Van der Loos H (1985) An electrophysiological and anatomical study of projections to the mouse cortical barrelfield and its surroundings. J Neurophysiol 53: 686–698
Price TR, Webster KE (1972) The cortico-thalamic projection from the primary somatosensory cortex of the rat. Brain Res 44: 636–640
Rice FL, Van der Loos H (1977) Development of the barrels and barrel field in the somatosensory cortex of the mouse. J Comp Neurol 171: 545–560
Rinvik E (1972) Organization of thalamic connections from motor and somatosensory cortical areas in cat. In: Frigyesi TL, Rinvik F, Yahr MB (eds) Corticothalamic projections and sensorimotor activities. Raven Press, New York, pp 57–88
Rustioni A, Schmechel DE, Spreafico R, Cuénod M (1983) Excitatory and inhibitory aminoacid putative neurotransmitters in the ventralis posterior complex: an autoradiographic and immunocytochemical study in rats and cats. In: Macchi G, Rustioni A, Spreafico R (eds) Somatosensory integration in the thalamus. Elsevier, Amsterdam, pp 365–383
Saporta S, Kruger L (1977) The organization of thalamocortical relay neurons in the rat ventrobasal complex studied by the retrograde transport of horseradish peroxidase. J Comp Neurol 174: 187–208
Scheibel ME, Scheibel AB (1966) Patterns of organization in specific and nonspecific thalamic fields. In: Purpura DP, Yahr MB (eds) The thalamus. Raven Press, New York, pp 13–46
Scheibel ME, Scheibel AB, Davis TH (1972) Some substrate of centrifugal control of thalamic cell ensemble. Corticothalamic projections and sensorimotor activities. In: Frigyesi TL, Rinvik F, Yahr MB (eds) Corticothalamic projections and sensorimotor activities. Raven Press, New York, pp 131–160
Sharp FR, Evans K (1982) Regional (14C)2-deoxyglucose uptake during vibrissae movements by rat motor cortex stimulation. J Comp Neurol 208: 255–287
Simons DJ (1978) Response properies of vibrissa units in rat SI somatosensory neocortex. J Neurophysiol 41: 798–820
Slotnick BM, Leonard CM (1975) A stereotaxic atlas of the albino mouse forebrain. US Department of Health, Education, and Welfare, Rockville, Maryland
Smith RL (1973) The ascending fiber projections from the principal sensory trigeminal nucleus in the rat. J Comp Neurol 148: 423–446
Spreafico R, Whitsel BL, Rustioni A, McKenna TM (1983) The organization of nucleus ventralis posterolateralis (VPL) of the cat and its relationship to the forelimb representation in cerebral cortical area SI. In: Macchi G, Rustioni A, Spreafico R (eds) Somatosensory integration in the thalamus. Elsevier, Amsterdam, pp 287–307
Sternberger LA (1979) Immunocytochemistry. John Wiley&Sons, New York
Van der Loos H (1976) Barreloids in mouse somatosensory thalamus. Neurosci Lett 2: 1–6
Van der Loos H, Welker E, Dörfl J, Rumo G (1986) Selective breeding for variations in patterns of mystacial vibrissae of mice; bilaterally symmetrical strains derived from ICR-stock. J Hered 77: 66–82
Van der Loos H, Woolsey TA (1973) Somatosensory cortex: structural alterations following early injury to sense organs. Science 179: 395–398
Waite PME (1973) Somatotopic organization of vibrissal responses in the ventro-basal complex of the rat thalamus. J Physiol (Lond) 228: 527–540
Welker C (1971) Microelectrode delineation of fine grain somatotopic organization of SmI cerebral neocortex in albino rat. Brain Res 26: 259–275
Welt C, Steindler DA (1977) Somatosensory cortical barrels and thalamic barreloids in reeler mutant mice. Neuroscience 2: 755–766
White EL (1978) Identified neurons in mouse SmI cortex which are postsynaptic to thalamocortical axons: a combined Golgi-electronmicroscopic and degeneration study. J Comp Neurol 181: 627–662
White EL (1979) Thalamocortical synaptic relations: a review with emphasis on the projections of specific thalamic nuclei to the primary sensory areas of the neocortex. Brain Res Rev 1: 275–311
White EL, DeAmicis RA (1977) Afferent and efferent projections of the region in mouse SmI cortex which contains the posteromedial barrel subfield. J Comp Neurol 175: 455–482
Wise SP, Jones EG (1977) Cells of origin and terminal distribution of descending projections of the rat somatic sensory cortex. J Comp Neurol 175: 129–158
Wise SP, Jones EG (1978) Developmental studies of thalamocortical and commissural connections in the rat somatic sensory cortex. J Comp Neurol 178: 187–208
Woolsey TA, Van der Loos H (1970) The structural organization of layer IV in the somatosensory region (SI) of mouse cerebral cortex. The description of a cortical field composed of discrete cytoarchitectonic units. Brain Res 17: 205–242
Yamakado M (1985) Postnatal development of barreloid neuropils in the ventrobasal complex of mouse thalamus: a histochemical study for cytochrome oxidase. Brain Nerve 37: 497–506
Yen C-T, Jones EG (1983) Intracellular staining of physiologically identified neurons and axons in the somatosensory thalamus of the cat. Brain Res 280: 148–154
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Hoogland, P.V., Welker, E. & Van der Loos, H. Organization of the projections from barrel cortex to thalamus in mice studied with Phaseolus vulgaris-leucoagglutinin and HRP. Exp Brain Res 68, 73–87 (1987). https://doi.org/10.1007/BF00255235
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00255235