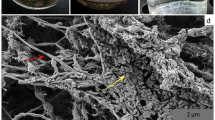
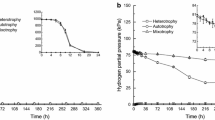
Abstract
Three different defined cocultures of glycolatedegrading strictly anaerobic bacteria were isolated from enrichment cultures inoculated with freshwater sediment samples. Each culture contained a primary fermenting bacterium which used only glycolate as growth substrate. These cells were gram-positive, formed terminal oval spores, and did not contain cytochromes. Growth with glycolate was possible only in coculture with either a homoacetogenic bacterium or a hydrogen-utilizing methanogenic bacterium; the overall fermentation balance was either 4 glycolate → 3 acetate + 2CO2, or 4 glycolate → 3 CH4+5 CO2. These transformations indicate that glycolate was converted by the primary fermenting bacterium entirely to CO2 and reducing equivalents which were transferred to the partner organisms, probably through interspecies hydrogen transfer. The key enzymes of fermentative glycolate degradation were identified in cell-free extracts. An acetyl-CoA and ADP-dependent glyoxylate-converting enzyme activity, malic enzyme, pyruvate synthase, and methyl viologen-dependent hydrogenase were found at comparably high activities suggesting that these bacteria oxidize glycolate through a new pathway via malyl-CoA, and that ATP is synthesized by substrate-level phosphorylation, in a similar manner as found in a recently isolated glyoxylatefermenting anaerobe.
Similar content being viewed by others
References
Anderson RL, Bishop WE, Campbell RLL (1985) A review of the environmental and mammalian toxicology of nitrilotriacetic acid. CRC Crit Rev Toxicol 15:1–102
Bateson MM, Ward MW (1988) Photoexcretion and fate of glycolate in a hot spring cyanobacterial mat. Appl Environ Microbiol 54:1738–1743
Beck E (1979) Glycolate synthesis. In: Pirson A, Zimmerman MH (eds) Photosynthesis II encyclopedia of plant physiology, New Series, vol. 6. Springer, Berlin Heidelberg New York, pp 327–335
Beudeker RF, Kuenen JG, Codd GA (1981) Glycollate metabolism in the obligate chemolithotroph Thiobacillus neapolitanus grown in continuous culture. J Gen Microbiol 126:337–346
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Codd GA, Bowien B, Schlegel HG (1976) Glycollate production and excretion by Alcaligenes eutrophus. Arch Microbiol 110:167–171
Conrad R, Wetter B (1990) Influence of temperature on energetics of hydrogen metabolism in homoacetogenic, methanogenic and other anaerobic bacteria. Arch Microbiol 155:94–98
Cord-Ruwisch R, Seitz H-J, Conrad R (1988) The capacity of hydrogenotrophic anaerobic bacteria to compete for traces of hydrogen depends on the redox potential of the terminal electron acceptor. Arch Microbiol 149:350–357
Dehning I, Stieb M, Schink B (1989) Sporomusa malonica sp. nov., a homoacetogenic bacterium growing by decarboxylation of malonate or succinate. Arch Microbiol 151:421–426
Diekert GB, Thauer RK (1978) Carbon monoxide oxidation by Clostridium thermoaceticum and Clostridium formicoaceticum. J Bacteriol 136:597–606
Dürre P, Andreesen JR (1982) Selenium-dependent growth and glycine fermentation by Clostridium purinolyticum. J Gen Microbiol 128:1457–1466
Dürre P, Spahr R, Andreesen JR (1983) Glycine fermentation via a glycine reductase in Peptococcus glycinophilus and Peptococcus magnus. Arch Microbiol 134:127–135
Edenborn HM, Litchfield CD (1985) Glycolate metabolism by Pseudomonas sp., strain S227, isolated from a coastal marine sediment. Mar Biol 88:199–205
Edenborn HM, Litchfield CD (1987) Glycolate turnover in the water column of the New York Bight apex. Mar Biol 95:459–467
Egli C, Thüer M, Suter D, Cook AM, Leisinger T (1989) Monochloro-and dichloroacetic acids as carbon and energy sources for a stable, methanogenic mixed culture. Arch Microbiol 152:218–223
Fogg GE, Nalewajko C, Watt WD (1965) Extracellular products of phytoplankton photosynthesis. Proc R Soc London B 162:517–534
Friedrich M, Schink B (1991) Fermentative degradation of glyoxylate by a new strictly anaerobic bacterium. Arch Microbiol 156:392–397
Gregersen T (1978) Rapid method for distinction of Gram-negative from Gram-positive bacteria. Eur J Appl Microbiol Biotechnol 5:123–127
Hansen RW, Hayashi JA (1962) Glycolate metabolism in Escherichia coli. J Bacteriol 83:679–687
Janssen PH (1990) Fermentation of glycollate by a mixed culture of anaerobic bacteria. Syst Appl Microbiol 13:327–332
Karrer W (1958) Konstitution und Vorkommen von organischen Pflanzenstoffen. Birkhäuser, Basel
Kornberg HL, Morris JG (1965) The utilization of glycollate by Micrococcus denitrificans: the beta-hydroxy-aspartate pathway. Biochem J 95:577–586
Kornberg HL, Sadler JR (1960) Microbial oxidation of glycollate via a dicarboxylic acid cycle. Nature 185:153–155
Kurz WGW, LaRue TAG (1973) Metabolism of glycolic acid by Azotobacter chroococcum PRL H62. Can J Microbiol 19:321–324
Magee CM, Rodeheaver G, Edgerton MT, Edlich RF (1975) A more reliable Gram staining technic for diagnosis of surgical infections. Am J Surgery 130:454–456
Odom JM, Peck HD (1981) Localisation of dehydrogenases, reductases and electron transfer components in the sulfate-reducing bacterium Desulfovibrio gigas. J Bacteriol 147:161–169
Pfennig N (1978) Rhodocyclus purpureus gen. nov. sp. nov., a ringshaped, vitamin B12-requiring member of the family Rhodospirillaceae. Int J Syst Bacteriol 28:283–288
Schauer R (1982) Chemistry, metabolism, and biological functions of sialic acids. Adv Carbohydr Chem Biochem 40:131–234
Schink B (1991) Syntrophism among prokaryotes. In: Balows A, Trüper HG, Dworkin M, Harder W, Schleifer KH (eds) The prokaryotes. Springer, New York Berlin Heidelberg (in press)
Schink B, Bomar M (1991) The genera Acetobacterium, Acetogenium, Acetoanaerobium, and Acetitomaculum. In: Balows A, Trüper HG, Dworkin M, Harder W, Schleifer KH (eds) The prokaryotes. Springer, New York Berlin Heidelberg (in press)
Schink B, Pfennig N (1982) Fermentation of trihydroxybenzenes by Pelobacter acidigallici gen. nov. spec. nov., a new strictly anaerobic, non-sporeforming bacterium. Arch Microbiol 133:195–201
Seitz HJ, Schink B, Pfennig N, Conrad R (1990) Energetics of syntrophic ethanol oxidation in defined chemostat cocultures. 1. Energy requirement for H2 production and H2 oxidation. Arch Microbiol 155:82–88
Shorey EC (1899) Glycollic acid: one of the acids of sugar-cane. J Am Chem Soc 21:45–50
Sneath PHA, Mair NS, Sharpe ME, Holt JG (eds) (1986) Bergey's manual of systematic bacteriology, vol. 2. Williams & Wilkins, Baltimore London Los Angeles
Stadtman TC (1980) Selenite-dependent enzymes. Ann Rev Biochem 49:93–110
Stams AJM, Kremer DR, Nicolay K, Weenk GH, Hansen TA (1984) Pathway of propionate formation in Desulfobulbus propionicus. Arch Microbiol 139:167–173
Stewart R, Codd GA (1981) Glycollate and glyoxylate excretion by Sphaerocystis schroeteri (Chlorophyceae). Br Phycol J 16:177–182
Stieb M, Schink B (1987) Cultivation of syntrophic anaerobic bacteria in membrane-separated culture devices. FEMS Microbiol Ecol 45:71–76
Tanaka K, Mikami E, Suzuki T (1986) Methane fermentation of 2-methoxyethanol by mesophilic digesting sludge. J Ferment Technol 64:305–309
Thauer RK, Morris JG (1984) Metabolism of chemotrophic anaerobes: old view and news aspects. In: Kelly DP, Carr NG (eds) The microbe 1984 part 2. Soc. Gen. Microbiol. Symp, vol. 36, pp. 123–168, Cambridge University Press, Cambridge
Thauer RK, Jungermann K, Decker K (1977) Energy conservation of chemotrophic anaerobic bacteria. Bacteriol Rev 41:100–180
Tolbert NE, Zill LP (1956) Excretion of glycolic acid by algae during photosynthesis. J Biol Chem 222:895–906
Tschech A, Pfennig N (1984) Growth yield increase linked to caffeate reduction in Acetobacterium woodii. Arch Microbiol 137:163–167
Tuboi S, Kikuchi G (1965) Enzymic cleavage of malyl-coenzyme A into acetyl-coenzyme A and glyoxylic acid. Biochim Biophys Acta 96:148–153
Watt WD (1966) Release of dissolved organic material from the cells of phytoplankton populations. Proc R Soc London [A] 164:521–551
Whittingham CP, Pritchard GG (1963) The production of glycollate during photosynthesis in Chlorella. Proc R Soc London [B] 157:366–380
Widdel F, Pfennig N (1981) Studies on dissimilatory sulfate-reducing bacteria that decompose fatty acids. I. Isolation of new sulfate reducing bacteria enriched with acetate from saline environments. Description of Desulfobacter postgatei gen. nov., sp. nov. Arch Microbiol 129:395–400
Widdel F, Kohring GW, Mayer F (1983) Studies on dissimilatory sulfate-reducing bacteria that decompose fatty acids. III. Charaterization of the filamentous gliding Desulfonema limicola gen. nov. sp. nov., and Desulfonema magnum sp. nov. Arch Microbiol 134:286–294
Windholz M, Budavri S, Blumetti RF, Otterbein ES (eds) (1983) The Merck Index, 10th edn. Merck, Rahway, NJ, USA
Wright RT (1975) Studies on glycolic acid metabolism by freshwater bacteria. Limnol Oceanogr 20:626–633
Wright RT, Shah NM (1975) The trophic role of glycolic acid in coastal seawater. I. Heterotrophic metabolism in seawater and bacterial cultures. Mar Biol 33:175–183
Wright RT, Shah NM (1977) The trophic role of glycolic acid in coastal seawater. II. Seasonal changes in concentration and heterotrophic use in Ipswich Bay, Massachusetts, USA. Mar Biol 43:257–263
Zhao H, Yang D, Woese CR, Bryant MP (1990) Assignment of Clostridium bryantii to Syntrophosphora bryantii gen. nov., com. nov., on the basis of a 16S rRNA sequece analysis of its crotonate-grown pure culture. Int J Syst Bacteriol 40:40–44
Zindel U, Freudenberg W, Rieth M, Andreesen JR, Schnell J, Widdel F (1988) Eubacterium acidaminophilum nov. sp., a versatile amino acid-degrading anaerobe producing or utilizing H2 or formate. Description and enzymatic studies. Arch Microbiol 150:254–266
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Friedrich, M., Laderer, U. & Schink, B. Fermentative degradation of glycolic acid by defined syntrophic cocultures. Arch. Microbiol. 156, 398–404 (1991). https://doi.org/10.1007/BF00248717
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00248717