Abstract
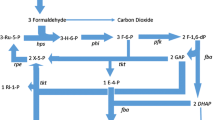
The thermotolerant methylotroph Bacillus sp. C1 possesses a novel NAD-dependent methanol dehydrogenase (MDH), with distinct structural and mechanistic properties. During growth on methanol and ethanol, MDH was responsible for the oxidation of both these substrates. MDH activity in cells grown on methanol or glucose was inversely related to the growth rate. Highest activity levels were observed in cells grown on the C1-substrates methanol and formaldehyde. The affinity of MDH for alcohol substrates and NAD, as well as V max, are strongly increased in the presence of a M r 50,000 activator protein plus Mg2+-ions [Arfman et al. (1991) J Biol Chem 266: 3955–3960]. Under all growth conditions tested the cells contained an approximately 18-fold molar excess of (decameric) MDH over (dimeric) activator protein. Expression of hexulose-6-phosphate synthase (HPS), the key enzyme of the RuMP cycle, was probably induced by the substrate formaldehyde. Cells with high MDH and low HPS activity levels immediately accumulated (toxic) formaldehyde when exposed to a transient increase in methanol concentration. Similarly, cells with high MDH and low CoA-linked NAD-dependent acetaldehyde dehydrogenase activity levels produced acetaldehyde when subjected to a rise in ethanol concentration. Problems frequently observed in establishing cultures of methylotrophic bacilli on methanol- or ethanol-containing media are (in part) assigned to these phenomena.
Similar content being viewed by others
Abbreviations
- MDH:
-
NAD-dependent methanol dehydrogenase
- ADH:
-
NAD-dependent alcohol dehydrogenase
- A1DH:
-
CoA-linked NAD-dependent aldehyde dehydrogenase
- HPS:
-
hexulose-6-phosphate synthase
- G6Pdh:
-
glucose-6-phosphate dehydrogenase
References
Al-Awadhi N, Egli T, Hamer G (1988) Growth characteristics of a thermotolerant methylotrophic Bacillus sp. (NCIMB 12522) in batch culture. Appl Microbiol Biotechnol 29: 485–493
Al-Awadhi N, Egli T, Hamer G, Wehrli E (1989) Thermotolerant and thermophilic solvent-utilizing methylotrophic aerobic bacteria. Syst Appl Microbiol 11: 207–216
Anthony C (1982) The biochemistry of methylotrophs. Academic Press, London
Arfman N, Watling EM, Clement W, Oosterwijk RJvan, Vries GEde, Harder W, Attwood MM, Dijkhuizen L (1989) Methanol metabolism in thermotolerant methylotrophic Bacillus strains involving a novel catabolic NAD-dependent methanol dehydrogenase as a key enzyme. Arch Microbiol 152: 280–288
Arfman N, Bystrykh LV, Govorukhina NI, Dijkhuizen L (1990) 3-Hexulose-6-phosphate synthase from the thermotolerant methylotroph Bacillus sp. C1. Methods Enzymol 188: 391–397
Arfman N, Beeumen Jvan, Vries GEde, Harder W, Dijkhuizen L (1991a) Purification and characterization of an activator protein for methanol dehydrogenase from thermotolerant Bacillus spp. J Biol Chem 266: 3955–3960
Arfman N, Frank J, Bystrykh LV, Govorukhina NI, Hektor HJ, Dijkhuizen L (1991 b) Kinetic properties of a novel NAD(H)-containing methanol dehydrogenase from thermotolerant Bacillus spp.: involvement of a Mr 50,000 activator protein, NAD and Mg2+. (in preparation)
Arfman N, Dijkhuizen L, Kirchhof G, Ludwig W, Schleifer K-H, Bulygina ES, Govorukhina NI, Chumakov KM, Trotsenko YA, White D, Sharp R (1992) Bacillus methanolicus sp. nov., a new species of thermotolerant methanol-utilizing endospore-forming bacteria. Int J Syst Bacteriol (in press)
Avigad G (1983) A simple spectrophotometric determination of formaldehyde and other aldehydes: application to periodate-oxidized glycol systems. Anal Biochem 134: 499–504
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72: 248–254
Brändén CI, Jörnvall H, Eklund H, Furugren B (1975) Alcohol dehydrogenases. In: Boyer PD (ed) The enzymes, 3rd edn, vol 11A. Academic Press, New York, pp 103–190
Brooke AG, Attwood MM, Tempest DW (1990) Metabolic fluxes during growth of thermotolerant methylotrophic Bacillus strains in methanol-sufficient chemostat cultures. Arch Microbiol 153: 591–595
Dijkhuizen L, Arfman N, Attwood MM, Brooke AG, Harder W, Watling EM (1988) Isolation and initial characterization of thermotolerant methylotrophic Bacillus strains. FEMS Microbiol Lett 52: 209–214
Govorukhina NI, Trotsenko YA, Suzina NE, Bulygina AS, Chumakov KM (1992) Isolation and characterization of thermotolerant methylotrophic bacilli. Microbiologya (in Russian) (in press)
Jörnvall H, Persson B, Jeffery J (1987) Characteristics of alcohol/polyol dehydrogenases. Eur J Biochem 167: 195–201
Levering PR, Dijken JPvan, Veenhuis M, Harder W (1981) Arthrobacter P1, a fast growing versatile methylotroph with amine oxidase as a key enzyme in the metabolism of methylated amines. Arch Microbiol 129: 72–80
Levering PR, Croes LM, Dijkhuizen L (1985) Regulation of methylamine and formaldehyde metabolism in Arthrobacter P1. Formaldehyde is the inducing signal for the synthesis of the RuMP cycle enzyme hexulose phosphate synthase. Arch Microbiol 144: 272–278
Roitsch T, Stolp H (1985) Overproduction of methanol dehydrogenase in glucose grown cells of a restricted RuMP type methylotroph. Arch Microbiol 142: 34–39
Roitsch T, Stolp H (1986) Synthesis of dissimilatory enzymes of serine type methylotrophs under different growth conditions. Arch Microbiol 144: 245–247
Schendel FJ, Bremmon CE, Flickinger MC, Guettler M, Hanson RS (1990) l-Lysine production at 50°C by mutants of a newly isolated and characterized methylotrophic Bacillus sp. Appl. Environ Microbiol 56: 963–970
Sund H, Theorell H (1963) Alcohol dehydrogenases. In: Boyer PD, Lardy H, Myrbäck K (eds) The enzymes, 2nd edn, vol 7A. Academic Press, New York, pp 25–83
Vonck J, Arfman N, Vries GEde, Beeumen Jvan, Bruggen EFJvan, Dijkhuizen L (1991) Electron microscopic analysis and biochemical characterization of a novel methanol dehydrogenase from the thermotolerant Bacillus sp. C1. J Biol Chem 266: 3949–3954
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Arfman, N., de Vries, K.J., Moezelaar, H.R. et al. Environmental regulation of alcohol metabolism in thermotolerant methylotrophic Bacillus strains. Arch. Microbiol. 157, 272–278 (1992). https://doi.org/10.1007/BF00245161
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00245161