Abstract
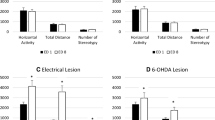
The present experiment assessed the locomotor response to a low dose (1 mg/kg) of systemic gemd-amphetamine in rats with cytotoxic lesions of the retrohippocampus (entorhinal and extra-subicular cortices), compared with vehicle-operated shams and unoperated controls. Under spontaneous and saline conditions, both the sham and the lesioned animals were more active than unoperated controls, and they did not differ from each other. Systemic gemd-amphetamine produced increased locomotion in all groups, but this effect was potentiated in animals with retrohippocampal lesions; two control groups did not differ from each other in their response to the drug. The present results are consistent with the suggestion that cell loss within the retrohippocampal region could affect the functional response of nucleus accumbens to amphetamine. The results are discussed in terms of the interaction between the retrohippocampus and nucleus accumbens in the control of mesolimbic dopamine release and the possible implications for schizophrenia.
Similar content being viewed by others
References
Brog JS, Salyapongse A, Deutch AY, Zahm DS (1993) The patterns of afferent innervation of the core and shell in the “accumbens” part of the rat ventral striatum: immunohistochemical detection of retrograde transported fluoro-gold. J Comp Neurol 338:255–278
Burns L, Robbins TW, Everitt BJ (1993) Differential effects of excitotoxic lesions of the basolateral amygdala, ventral subiculum and medial prefrontal cortex on responding with conditioned reinforcement and locomotory activity potentiated by intra-accumbens infusion of gemd-amphetamine. Behav Brain Res 55:167–183
Creese I, Iversen SD (1975) The pharmacological and anatomical substrates of the amphetamine response in the rat. Brain Res 83:419–436
Csernansky JG, Murphy GM, Faustman WO (1991) Limbic/mesolimbic connections and the pathogenesis of schizophrenia. Biol Psychiatr 30:383–400
Danscher G (1981) Histochemical demonstration of heavy metals: a revised version of the sulphide silver method suitable for both light and electromicroscopy. Histochemistry 71:1–16
DeFrance JF, Marhand JF, Stanley J, Sikes RW, Chronister RB (1980) Convergence of excitatory amygdaloid and hippocampal input in the nucleus accumbens septi. Brain Res 185:183–186
DiChiara G, Tanda G, Fray R, Carboni E (1993) On the preferential release of dopamine in the nucleus accumbens by amphetamine: further evidence obtained by vertically implanted concentric dialysis probes. Psychopharmacology 112:398–402
Donzanti BA, Uretsky NJ (1983) Effects of excitatory amino acids on locomotor activity after bilateral microinjection into the rat nucleus accumbens: possible dependence on dopaminergic mechanism. Neuropharmacology 22:971–981
Essam WD, McGonigle P, Lucki I (1993) Anatomical differentiation within the nucleus accumbens of the locomotor stimulatory actions of selective dopamine agonists and gemd-amphetamine. Psychopharmacology 112:233–241
Fass B (1983) Temporal changes in open-field activity following progressive lesions of entorhinal cortex: evidence for enhanced recovery. Behav Neural Biol 37:108–124
Frederickson CJ, Danscher G (1989) Zinc-containing neurons in the hippocampus and related structures. Progr Brain Res 83:71–84
Frederickson CJ, Klitenick MA, Manton WI, Kirkpatrick JB (1983) Cytoarchitectonic distribution of zinc in the hippocampus of man and the rat. Brain Res 273:335–339
Friston KJ, Liddle PF, Frith CD, Hirsch SR, Frackowiak RSJ (1993) The left medial temporal region and schizophrenia. Brain 115:367–382
Frotscher M, Zimmer J (1983) Lesion-induced mossy fibres to the molecular layer of the fascia dentata: identification of postsynaptic granule cells by the Golgi-EM technique. J Comp Neurol 215:299–311
Geneser FA, Haug FMS, Danscher G (1974) Distribution of heavy metals in the hippocampal region of the guinea pig. A light microscopic study with the Timm's sulphide silver method. Z Zellforch 147:441–478
Gray JA, Feldon J, Rawlins JNP, Hemsley DR, Smith AD (1991) The neuropsychology of schizophrenia. Behav Brain Sci 14:1–84
Groenewegen HJ, Room P, Witter MP, Lohman AMH (1982) Cortical afferents of the nucleus accumbens in the cat studied with anterograde and retrograde transport techniques. Neuroscience 7:977–996
Groenewegen HJ, Vermeulen-Van der Zee E, Te Kortschot A, Witter MP (1987) Organization of the projections from the subiculum to the ventral striatum in the rat. A study using anterograde transport of PHA-L. Neuroscience 23:103–120
Hagan JJ, Verheijck EE, Spigt MH, Ruigt GSF (1992) Behavioural and electrophysiological studies of entorhinal cortex lesions in the rat. Physiol Behav 51:255–266
Hannigan JH, Springer JE, Isaacson RE (1984) Differentiation of basal ganglia dopaminergic involvement in behaviour after hippocampectomy. Brain Res 291:83–91
Haug FMS (1974) Light microscopic mapping of the hippocampal region, the piriform cortex and the corticomedial amygdaloid nuclei of the rat with Timm's sulphide silver method. Z Anat Entwickl Gesch 145:1–27
Hedreen JC, Bacon SJ, Price DL (1985) A modified histochemical technique to visualize acetylcholinesterase-containing axons. J Histochem Cytochem 33:134–140
Isaacson RL (1984) Hippocampal damage: effects on dopaminergic systems of the basal ganglia. Int Rev Neurobiol 25:339–359
Jarrard LE (1968) Behaviour of hippocampal lesioned rats in home cage and novel situations. Physiol Behav 3:65–70
Kelly AE, Domesick VB (1982) The distribution of the projection from the hippocampal formation to the nucleus accumbens in the rat: an anterograde and retrograde-HRP study. Neuroscience 7:2321–2335
Killcross AS, Robbins TW (1993) Differential effects of intra-accumbens and systemic amphetamine on latent inhibition using an on-baseline, within-subject conditioned suppression paradigm. Psychopharmacology 110:479–489
Köhler C (1985) Intrinsic projections of the retrohippocampal region in the rat brain. I. The subicular complex. J Comp Neurol 236:504–522
Köhler C, Sundberg H (1977) Locomotor activity and exploratory behaviour after medial entorhinal cortex lesions in the albino rat. Behav Biol 20:419–432
Lasher SS, Steward O (1981) The time course of changes in open ield activity following bilateral entorhinal lesions in rats and cats. Behav Neural Biol 32:1–20
Laurberg S, Zimmer J (1981) Lesion-induced sprouting of hippocampal mossy fibre collaterals to the fascia dentata in adult rats. J Comp Neurol 200:433–459
Liddle PF, Friston KJ, Frith CD, Jones T, Hirsch SR, Frackowiak RSJ (1992) Patterns of cerebral blood flow in schizophrenia. Br J Psychiatr 160:179–186
Lynch G, Matthews DA, Mosko S, Parks T, Cotman CW (1972) Induced acetylcholinesterase-rich layer in rat dentate gyrus following entorhinal lesions. Brain Res 42:311–318
Mittleman G, LeDuc PA, Whishaw IQ (1993) The role of D1 and D2 receptors in the heightened locomotion induced by direct and indirect dopamine agonists in rats with hippocampal damage: an animal analogue of schizophrenia. Behav Brain Res 55:253–267
Newman R, Winans SS (1980) An experimental study of the ventral striatum of the golden hamster. I. Neuronal connections of the nucleus accumbens. J Comp Neurol 191:167–192
Paxinos G, Watson C (1986) The rat brain in stereotaxic coordinates, 2nd edn. Academic Press, New York
Pennartz CMA, Groenewegen HJ, Lopes da Silva FH (1994) The nucleus accumbens as a complex of functionally distinct neuronal ensembles: an integration of behavioural, electrophysiological and anatomical data. Prog Neurobiol 42:719–761
Pennartz CMA, Kitai ST (1991) Hippocampal inputs to identified neurons in an in vivo slice preparation of the rat nucleus accumbens: evidence for feed-forward inhibition. J Neurosci 11:2838–2847
Pijnenburg AJJ, Honig WMM, Van der Heyden JAM, van Rossum JM (1976) Effects of chemical stimulation of the mesolimbic dopamine system upon locomotor activity. Eur J Pharmacol 35:45–58
Roberts GW (1991) Schizophrenia: a neuropathological perspective. Br J Psychiatr 158:8–17
Roberts GW, Horton K (1992) Neuropathology of psychoses: towards a common biology. In: Trimble MR, Bolwig TG (eds) The temporal lobes and the limbic system. Wrightson Biomedical, Petersfield, UK, pp 213–238
Robinson TE, Becker JB (1986) Enduring changes in brain and behaviour produced by chronic amphetamine administration: a review and evaluation of animal models of amphetamine psychosis. Brain Res Rev 11:157–198
Ross JF, Walsh LL, Grossman SP (1973) Some behavioural effects of entorhinal cortex lesions in albino rats. J Comp Physiol Psychol 85:70–81
Sesack SR, Pickel VM (1990) In the rat medial nucleus accumbens, hippocampal and catecholaminergic terminals converge on spiny neurons and are in opposition to each other. Brain Res 527:266–279
Shreve PE, Uretsky NJ (1988) Role of quisqualic acid receptors in the hypermotility response produced by the injection of AMPA into the nucleus accumbens. Pharmacol Biochem Behav 30:379–384
Solomon PR, Staton DM (1982) Differential effects of microinjections of gemd-amphetamine into the nucleus accumbens or the caudate putamen on the rat's ability to ignore an irrelevant stimulus. Biol Psychiatr 17:743–756
Sørensen KE, Witter MP (1983) Entorhinal efferents reach the caudato-putamen. Neurosci Lett 35:259–264
Springer HE, Isaacson RL (1982) Catecholamine alterations in basal ganglia after hippocampal lesions. Brain Res 252:185–188
Stanfield BB, Cowan WM (1982) The sprouting of septal afferents to the dentate gyrus after lesions of the entorhinal cortex in adult rats. Brain Res 232:162–170
Steward O, Loesche J, Horton WC (1977) Behavioral correlates of denervation and reinnervation of the hippocampal formation of the rat: open field activity and cue utilization following bilateral entorhinal cortex lesions. Brain Res Bull 2:41–48
Teitelbaum H, Milner P (1963) Activity changes following partial hippocampal lesions in rats. J Comp Physiol Psychol 56:284–289
Totterdell S, Smith AD (1989) Convergence of hippocampal and dopaminergic input onto identified neurons in the nucleus accumbens of the rat. J Chem Neuroanat 2:285–298
Weiner I, Lubow RE, Feldon J (1988) Disruption of latent inhibition by acute administration of low doses of amphetamine. Pharmacol Biochem Behav 30:871–878
West JR, Dewey SL (1984) Mossy fibre sprouting in the fascia dentata after unilateral entorhinal lesions: quantitative analysis using computer-assisted image processing. Neuroscience 13:377–384
Whishaw IQ, Mittleman G (1991) Hippocampal modulation of nucleus accumbens: behavioural evidence from amphetamine-induced activity profile. Behav Neural Biol 55:289–306
Wilkinson LS, Mittleman G, Torres E, Humby T, Hall FS, Robbins TW (1993) Enhancement of amphetamine-induced locomotor activity and dopamine release in nucleus accumbens following excitotoxic lesion of the hippocampus. Behav Brain Res 55:143–150
Witter MP (1989) Connectivity of the rat hippocampus. In: Chan-Palay V, Köhler C (eds) The hippocampus: new vista. (Neurology and neurobiology, vol 52) Liss, New York, pp 53–69
Witter MP, Groenewegen HJ (1992) Organization principles of hippocampal connections. In: Trimble MR, Bolwig TG (eds) The temporal lobes and the limbic system. Wrightson Biomedical, Petersfield, UK, pp 37–60
Witter MP, Groenewegen HJ, Lopes da Silva FH, Lohman AHM (1989) Functional organization of the extrinsic and intrinsic circuitry of the parahippocampal region. Prog Neurobiol 33:161–253
Yang CR, Mogenson GJ (1987) Hippocampal signal transmission to the pedunculopontine nucleus and its regulation by dopamine D2 receptors in the nucleus accumbens: an electrophysiological and behavioural study. Neuroscience 23:1041–1055
Yee BK, Feldon J, Rawlins JNP (1995) Latent inhibition in rats is abolished by NMDA-induced retrohippocampal cell loss but this lesion effect can be prevented by systemic haloperidol treatment. Behav Neurosci 109:227–240
Yung KKL, Bolam JP, Smith AD, Ciliax BJ, Levey AI (1995) Immunocytochemical localisation of D1 and D2 dopamine receptors in the basal ganglia of the rat: light and electron microscopy. Neuroscience 65:709–730
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Yee, B.K., Feldon, J. & Rawlins, J.N.P. Potentiation of amphetamine-induced locomotor activity following NMDA-induced retrohippocampal neuronal loss in the rat. Exp Brain Res 106, 356–364 (1995). https://doi.org/10.1007/BF00241131
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00241131