Summary
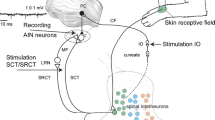
We report the connections of cerebellar cortical lobule HVI in the rabbit. We have studied the anterograde and retrograde transport of wheatgerm-agglutinated horseradish peroxidase (WGA-HRP) following its injection into HVI to reveal efferent and afferent connections. All of the cases showed strong anterograde transport to the anterior interpositus nucleus (AIP) — indicating that this is the major efferent target of HVI. Retrogradely labelled cells were found in the inferior olivary, spinal trigeminal, lateral reticular, inferior vestibular and pontine nuclei. Within the olive, the medial part of the rostral dorsal accessory olive (DAO) and the adjacent medial part of the principal olive (PO) were consistently labelled in all cases. This area is known to receive somatosensory information from the face and neck. There was no projection to the hemispheral part of lobule VI from visual parts of the olive within the dorsal cap and medial parts of the medial accessory olive. Likely sources of visual and auditory information to HVI are the dorsolateral basilar pontine nuclei and nucleus reticularis tegmenti pontis, which were densely labelled in all cases. These anatomical findings are consistent whith the suggestion that, during NMR conditioning, information related to the periorbital shock unconditional stimulus (US) may be provided by climbing fibres to HVI and light and white noise conditional stimulus (CS) information may be supplied by pontine mossy fibres.
Similar content being viewed by others
References
Albus JS (1971) A theory of cerebellar function. Math Biosci 10: 25–61
Altman J, Carpenter MB (1961) Fiber connections of the superior colliculus of the cat. J Comp Neurol 116: 157–178
Armstrong DM, Harvey RJ, Schild RF (1974) Topographical localization in the olivocerebellar projection: an electrophy-siological study in the cat. J Comp Neurol 154: 287–302
Berkley KJ, Hand PJ (1978) Projections to the inferior olive of the cat. II. Comparisons of input from the gracile, cuneate and spinal trigeminal nuclei. J Comp Neurol 180: 253–264
Bernard JF, Buisseret-Delmas C, Compoint C, Laplante S (1984) Harmaline induced tremor. III. A combined simple units, horseradish peroxidase, and 2-deoxyglucose study of the olivocerebellar system in the rat. Exp Brain Res 57: 128–137
Brodal A (1939) Experimentelle Untersuchungen über retrograde Zellveränderungen in der unteren Olive nach Läsionen des Kleinhirns. Z Ges Neurol Psychiat 166: 622–704
Brodal A (1940) Experimentelle Untersuchungen über die olivocerebellare Lokalisation. Z Ges Neurol Psychiat 169: 1–153
Brodal A, Jansen J (1946) The ponto-cerebellar projection in the rabbit and cat. Experimental investigations. J Comp Neurol 84: 31–118
Brodal A, Kawamura K (1980) Olivocerebelar projection: a review. Adv Anat Embryol Cell biol 64: 1–140
Buchtel HA, Iosif G, Marchesi GF, Provini L, Strata P (1972) Analysis of activity evoked in cerebellar cortex by stimulation of the visual pathways. Exp Brain Res 15: 278–288
Campbell NC, Armstrong DM (1983) Topographic localization in the olivocerebellar projection in the rat: an autoradiographic study. Brain Res 275: 235–249
Clark GA, McCormick DA, Lavond DG, Thompson RF (1984) Effects of lesions of cerebellar nuclei on conditioned behavioural and hippocampal neuronal responses. Brain Res 291: 125–136
Courville J (1975) Distribution of olivocerebellar fibers demonstrated by a radioautographic tracing method. Brain Res 95: 253–263
Courville J, Augustine JR, Martel P (1977) Projections from the inferior olive to the cerebellar nuclei in the cat demonstrated by retrograde transport of horseradish peroxidase. Brain Res 130: 405–419
Dietrichs E, Bjaalie JG, Brodal P (1983) Do pontocerebellar fibers send collaterals to the cerebellar nuclei? Brain Res 259: 127–131
Disterhoft JF, Kwan HH, Lo WD (1977) Nictitating membrane conditioning to tone in the immobilized albino rabbit. Brain Res 137: 127–143
Eccles JC, Ito M, Szentágothai J (1967) The cerebellum as a neuronal machine. Springer, Berlin
Ekerot CF, Larson B (1979) The dorsal spino-olivocerebellar system in the cat. I. Functional organisation and termination in the anterior lobe. Exp Brain Res 36: 201–217
Furber SE, Watson CR (1983) Organization of the olivocerebellar projection in the rat. Brain Behav Evol 22: 132–152
Gellman R, Houk JC, Gibson AR (1983) Somatosensory properties of the inferior olive of the cat. J Comp Neurol 215: 228–243
Gould BB (1980) Organisation of afferents from the brain stem nuclei to the cerebellar cortex in the cat. Adv Anat Embryol Cell Biol 62: 1–90
Groenewegen HJ, Voogd J (1977) The parasagittal zonation within the olivocerebellar projection. I. Climbing fiber distribution in the vermis of cat cerebellum. J Comp Neurol 174: 417–488
Groenewegen HJ, Voogd J, Freedman SL (1979) The parasagittal zonal organisation within the olivocerebellar projection. II. Climbing fiber distribution in the intermediate and hemispheric parts of cat cerebellum. J Comp Neurol 183: 551–602
Holstege G, Collewijn H (1984) The efferent connections of the nucleus of the optic tract and the superior colliculus in the rabbit. J Comp Neurol 209: 139–175
Ikeda M (1979) Projections from the spinal and the principal sensory nuclei of the trigeminal nerve to the cerebellar cortex in the cat, as studied by retrograde transport of horseradish peroxidase. J Comp Neurol 184: 567–586
Ikeda M, Matsushita M (1974) Electronmicroscopic observations on the olivary projections to the cerebellar nuclei in the cat. Experientia 30: 536–538
Ito M (1984) The cerebellum and neural control. Raven Press, New York, pp 268–271
Kawamura K (1975) The pontine projection from the inferior colliculus in the cat. An experimental anatomical study. Brain Res 95: 309–322
Kawamura K, Brodal A (1973) The tectopontine projection in the cat: an experimental anatomical study with comments on the pathways for teleceptive impulses to the cerebellum. J Comp Neurol 149: 371–390
Kawamura K, Brodal A, Hoddevik G (1974) The projection of the superior colliculus onto the reticular formation of the brainstem: an experimental anatomical study in the cat. Exp Brain Res 19: 1–19
Kotchabhakdi N, Walberg F, Brodal A (1978) The olivocerebellar projection in the cat studied with the method of retrograde transport of horseradish peroxidase. VII. The projection to lobulus simplex, crus I and crus II. J Comp Neurol 182: 293–314
Linauts M, Martin GF (1978) The organisation of olivo-cerebellar projections in the opossum, Didelphis virginiana, as revealed by the retrograde transport of horseradish peroxidase. J Comp Neurol 179: 355–382
Marr D (1969) A theory of cerebellar cortex. J Physiol (Lond) 202: 437–470
Matsushita M, Ikeda M (1970) Olivary projections to the cerebellar nuclei in the cat. Exp Brain Res 10: 488–500
Matsushita M, Ikeda M, Okado N (1982) The cells of origin of the trigeminothalamic, trigeminospinal and trigeminocerebellar projections in the cat. Neuroscience 7: 1439–1454
McCormick DA, Thompson RF (1984) Cerebellum: essential involvement in the classically conditioned eyelid response. Science 223: 296–299
McCrea RA, Bishop GA, Kitai ST (1977) Electrophysiological and horseradish peroxidase studies of precerebellar afferents to the nucleus interpositus anterior. II. Mossy fiber system. Brain Res 122: 215–228
Meesen H, Olzewski J (1949) A cytoarchitectonic atlas of the rhombencephalon of the rabbit. S Karger, Basel
Mesulam M-M (1982) Principles of horseradish peroxidase neurohistochemistry and their applications for tracing neural pathways — axonal transport, enzyme histochemistry and light microscopic analysis. In: Mesulam M-M (ed) Tracing neural connections with horseradish peroxidase. Wiley, Chichester, pp 1–151
Miles TS, Wiesendanger M (1975a) Organisation of climbing fibre projections to the cerebellar cortex from trigeminal cutaneous afferents and from the SI face area of the cat. J Physiol (Lond) 245: 409–424
Miles TS, Wiesendanger M (1975b) Climbing fibre inputs to cerebellar Purkinje cells from trigeminal cutaneous afferents and from the SI face area of the cerebral cortex in the cat. J Physiol (Lond) 245: 425–445
Ono M, Kato H (1938) Zur Kenntnis von den Kleinhirnkernen des Kaninchens. Anat Anz 86: 245–259
Oakley DA, Russell IS (1972) Neocortical lesions and Pavlovian conditioning in the rabbit. Physiol Behav 8: 915–926
Oakley DA, Russell IS (1977) Subcortical storage of Pavlovian conditioning in the rabbit. Physiol Behav 18: 931–937
Robinson FR, Cohen JL, May J, Sestokas AK, Glickstein M (1984) Cerebellar targets of visual pontine cells in the cat. J Comp Neurol 223: 471–482
Rosina A, Provini L (1982) Longitudinal and topographical organisation of the olivary projection in the cat ansiform lobule. Neuroscience 7: 2657–2676
Saint-Cyr JA, Courville J (1982) Descending projections to the inferior olive from the mesencephalon and superior colliculus in the cat. Exp Brain Res 45: 333–348
Skelton RW, Donegan NH, Thompson RF (1984) Superior colliculus lesions disrupt classical conditioning to visual but not auditory stimuli. Soc Neurosci Abstr 10: 132
Somana R, Kotchabhakdi N, Walberg F (1980) Cerebellar afferents from the trigeminal sensory nuclei in the cat. Exp Brain Res 38: 57–64
Terasawa K, Otani K, Yamada J (1979) Descending pathways of the nucleus of the optic tract in the rat. Brain Res 173: 405–417
Tsukahara N, Bando T, Murakami F, Oda Y (1983) Properties of cerebello-precerebellar reverberating circuits. Brain Res 274: 249–259
van Rossum J (1969) Corticonuclear and corticovestibular projections of the cerebellum. Ph. D. thesis, University of Leiden
Voogd J (1969) The importance of fiber connections in the comparative anatomy of the mammalian cerebellum. In: Llinás R (ed) Neurobiology of cerebellar evolution and development. Am Med Assoc, Chicago, pp 493–514
Voogd J (1983) Anatomical evidence for a cortical “X” zone in the cerebellum of the cat. Soc Neurosci Abstr 9: 1091
Voogd J, Bigaré F (1980) Topographical distribution of olivary and corticonuclear fibers in the cerebellum: a review. In: Courville J, de Montigny C, Lamarre Y (eds) The inferior olivary nucleus. Raven Press, New York, pp 207–234
Weber JT, Partlow GD, Harting JK (1978) The projection of the superior colliculus upon the inferior olivary complex of the cat: an autoradiographic and horseradish peroxidase study. Brain Res 144: 369–377
Yeo CH, Hardiman MJ, Glickstein M (1984) Discrete lesions of the cerebellar cortex abolish the classically conditioned nictitating membrane response of the rabbit. Behav Brain Res 13: 261–266
Yeo CH, Hardiman MJ, Glickstein M (1985a) Classical conditioning of the nictitating membrane response of the rabbit. I. Lesions of the cerebellar nuclei. Exp Brain Res 60: 87–98
Yeo CH, Hardiman MJ, Glickstein M (1985b) Classical conditioning of the nictitating membrane response of the rabbit. II. Lesions of the cerebellar cortex. Exp Brain Res 60: 99–113
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Yeo, C.H., Hardiman, M.J. & Glickstein, M. Classical conditioning of the nictitating membrane response of the rabbit. Exp Brain Res 60, 114–126 (1985). https://doi.org/10.1007/BF00237024
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00237024