Summary
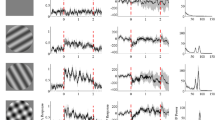
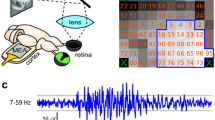
We have recorded extracellularly from single cells in area 19 of the cat for the purpose of providing a quantitative description of response characteristics. A prominent feature of this area is a high incidence of cells that are end-stopped. Drifting sinusoidal gratings were used to determine spatial and temporal characteristics of the discharge region. In addition, we have conducted independent tests to characterize end zones of receptive fields. When a grating patch was used to stimulate the discharge region alone, all of the cells showed a band-pass spatial frequency tuning characteristic. The optimal spatial frequency ranged from 0.1 to 1.13 cycles/deg, and the distribution had a peak at 0.4 cycles/deg. The bandwidth at half peak amplitude ranged widely from 0.7 to 3.3 octaves (mean 2.0 octaves). When gratings were also presented to the end zones, responses to stimulation of the central region were suppressed. The surround was phase-insensitive in that the relative phase between the grating in the two regions generally did not affect the strength of the suppression. To determine spatial characteristics of the end-zone inhibition, the spatial frequency of the end-zone grating was changed while that for the central pattern was fixed. All cells showed a bandpass characteristic for end-zone inhibition, but in each case, the tuning width was broader than that for excitation. The mean spatial frequency bandwidth of end-zone inhibition was 2.7 octaves. The peak of the inhibition generally coincided with the peak of the excitatory spatial frequency tuning of the discharge center. Considered together, these results show that neurons in area 19 share common properties with those in areas 17 and 18, but they exhibit phase-insensitve end-zone inhibition more frequently.
Similar content being viewed by others
References
DeValois RL, Albrecht DG, Thorell LG (1982) Spatial frequency selectivity of cells in macaque visual cortex. Vision Res 22: 545–559
Doty RW (1971) Survival of pattern vision after removal of striate cortex in the adult cat. J Comp Neurol 143: 341–370
Dreher B, Leventhal AG, Hale PT (1980) Geniculate input to cat visual cortex: a comparison of area 19 with areas 17 and 18. J Neurophysiol 44: 804–826
Duysens J, Orban GA, van der Glas HW, de Zegher FE (1982a) Functional properties of area 19 as compared to area 17 of the cat. Brain Res 231: 279–291
Duysens J, Orban GA, van der Glas HW, Maes H (1982b) Receptive field structure of area 19 as compared to area 17 of the cat. Brain Res 231: 293–308
Freeman RD, Robson JG (1982) A new approach to the study of binocular interaction of visual cortex: normal and monocular deprived cats. Exp Brain Res 48: 296–300
Gilbert CD, Kelly JP (1975) The projections of cells in different layers of the cat's visual cortex. J Comp Neurol 163: 81–106
Henry GH, Bishop PO, Coombs JS (1969) Inhibitory and subliminal excitatory receptive fields of simple units in cat striate cortex. Vision Res 9: 1289–1296
Hubel DH, Wiesel TN (1965) Receptive fields and functional architecture in two nonstriate visual areas (18 and 19) of the cat. J Neurophysiol 28: 229–289
Kimura M, Shiida T, Tanaka K, Toyama K (1980) Three classes of area 19 cortical cells of the cat classified by their neuronal connectivity and photic responsiveness. Vision Res 20: 60–77
Maciewicz RJ (1975) Thalamic afferents to areas 17, 18, and 19 of cat cortex traces with horseradish peroxidase. Brain Res 84: 308–312
Movshon JA, Thompson ID, Tolhurst DJ (1978a) Spatial summation in the receptive fields of simple cells in the cat's striate cortex. J Physiol (Lond) 283: 53–77
Movshon JA, Thompson ID, Tolhurst DJ (1978b) Receptive field organization of complex cells in the cat's striate cortex. J Physiol (Lond) 283: 79–99
Movshon JA, Thompson ID, Tolhurst DJ (1978c) Spatial and temporal contrast sensitivity of neurones in areas 17 and 18 of the cat's visual cortex. J Physiol (Lond) 283: 101–120
Morrone MC, Burr DC, Maffei L (1982) Functional implications of cross orientation inhibition of cortical visual cells. I. Neurophysiological evidence. Proc R Soc London B216: 335–354
Ohzawa I, Freeman RD (1986) Binocular organization of simple cells in the cat's visual cortex. J Neurophysiol 56: 221–242
Sanides F, Hoffman J (1969) Cyto- and myeloarchitecture of the visual cortex of the cat and of the surrounding integration cortices. J Hirnforsch 11: 79–104
Schiller RH, Finlay BL, Volman SF (1976) Quantitative studies of single-cell properties in monkey striate cortex. III. Spatial frequency. J Neurophysiol 39: 1334–1351
Sprague JM, Levy J, Diberardino A, Berlucchi G (1977) Visual cortical areas mediating form discrimination in the cat. J Comp Neurol 172: 441–488
Tusa RJ, Rosenquist AC, Palmer LA (1979) Retinotopic organization of areas 18 and 19 in the cat. J Comp Neurol 185: 657–678
Author information
Authors and Affiliations
Additional information
On leave from NHK Science and Technical Research Laboratories, Kinuta Setagaya, Tokyo 157, Japan
Rights and permissions
About this article
Cite this article
Tanaka, K., Ohzawa, I., Ramoa, A.S. et al. Receptive field properties of cells in area 19 of the cat. Exp Brain Res 65, 549–558 (1987). https://doi.org/10.1007/BF00235978
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00235978