Abstract
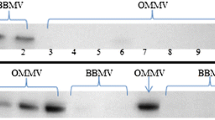
Fluorescein isothiocyanate (FITC) fluorescently labels amino groups and has been useful in detecting conformational changes in transport proteins through quenching or enhancement of the fluorescence signal upon exposure of protein to substrates. Solubilized renal basolateral membrane proteins, enriched in Na+/HCO −3 cotransporter activity, were reconstituted into liposomes and treated with FITC or its nonfluorescent analogue PITC (phenyl isothiocyanate). In the absence of Na+ and HCO −3 , incubation of proteoliposomes with PITC or FITC significantly inhibited cotransporter activity. However, in the presence of Na+ and HCO −3 during labeling both agents failed to inhibit cotransporter activity, indicating that these probes interact specifically with the cotransporter. In the presence of the substrates Na+ and HCO −3 , PITC binds covalently to amino groups unprotected by substrates leaving the Na+/HCO −3 cotransporter available for specific labeling with FITC. Addition of NaHCO3 to FITC-labeled proteoliposomes resulted in a concentration-dependent enhancement of the fluorescence signal which was inhibited by pretreatment with 4,4′-diisothiocyanostilbene 2′,2-disulfonic acid (DIDS) prior to FITC labeling. SDS PAGE analysis of FITC-treated proteoliposomes showed the presence of two distinct fluorescent bands (approximate MW of 90 and 56 kD). In the presence of substrates, the fluorescence intensity of these bands was enhanced as confirmed by direct measurement of gel slice fluorescence. Thus, FITC detects conformational changes of the Na+/HCO −3 cotransporter and labels proteins which may represent the cotransporter or components of this cotransporter.
This work was supported by the Merit Review Program from the Veterans Administration Central Office (J.A.L.A.), and the National Kidney Foundation of Illinois (A.A.B.).
Similar content being viewed by others
References
Akiba, T., Alpern, R.J., Eveloff, J., Calamina, J., Warnock, D.G. 1986. Electrogenic sodium/bicarbonate cotransport in rabbit renal cortical basolateral membrane vesicles. J. Clin. Invest. 78:1472–1478
Alpern, R.J., Chambers, M. 1986. Cell pH in the rat proximal convoluted tubule: regulation by luminal and peritubular pH and sodium concentration. J. Clin. Invest. 78:502–510
Bernardo, A.A., Kear, F.T., Ruiz, O.S., Arruda, J.A.L. 1994. Renal cortical basolateral Na+/HCO −3 cotransporter: I. Partial purification and reconstitution. J. Membrane Biol. 140:31–37
Boron, W.F., Boulpaep, E.L. 1983. Intracellular pH regulation in the renal proximal tubule of the salamander: basolateral HCO −3 transport. J. Gen. Physiol. 81:53–94
Grassl, S.M., Aronson, P.S. 1986. Na+/HCO −3 co-transport in basolateral membrane vesicles isolated form rabbit renal cortex. J. Biol. Chem. 261:8778–8783
Jackson, R.J., Mendlein, J., Sachs, G. 1983. Interaction of fluorescein isothiocyanate with the (H+ + K+)-ATPase. Biochim. Biophys. Acta 731:9–15
Karlish, S.J.D. 1980. Characterization of conformational changes in (Na,K) ATPase labeled with fluorescein at the active site. J Bioenerg. Biomembr. 12:111–136
Laemmli, M.K. 1970. Cleavage of structural proteins during assembly of the head of bacteriophage T4. Nature 227:680–685
Mitchison, C., Wilderspin, A.F., Trinnaman, B.J., Green, N.M. 1982. Identification of a labelled peptide after stoichiometric reaction of fluorescein isothiocyanate with the Ca2+-dependent adenosine triphosphatase of sarcoplasmic reticulum. FEBS Lett. 146:87–92
Peerce, B.E., Wright, E.M. 1984. Conformational changes in the intestinal brush border sodium-glucose cotransporter labeled with fluorescein isothiocyanate. Proc. Natl. Acad. Sci. USA 81:2223–2226
Pick, U. 1981. Interaction of fluorescein isothiocyanate with nucleotide-binding sites of the Ca-ATPase from sarcoplasmic reticulum. Eur. J. Biochem. 121:187–195
Pick, U., Bassilian, S. 1981. Modification of the ATP binding site of the Ca2+-ATPase from sarcoplasmic reticulum by fluorescein isothiocyanate. FEBS Lett. 123:127–136
Pick, U., Karlish, S.J.D. 1982. Regulation of the conformational transition in the Ca-ATPase from sarcoplasmic reticulum by pH, temperature, and calcium ions. J. Biol. Chem. 257:6120–6126
Ruiz, O.S., Arruda, J.A.L., Talor, Z. 1989. Na-HCO3 cotransport and Na-H antiporter in chronic respiratory acidosis and alkalosis. Am. J. Physiol. 256:F414–420
Sen, P.C., Kapakos, J.G., Steinberg, M. 1981. Modification of (Na++K+)-dependent ATPase by fluorescein isothiocyanate: Evidence for the involvement of different amino groups at different pH values. Arch. Biochem. Biophys. 211:652–661
Soleimani, M., Aronson, P.S. 1989. Effects of acetazolamide on Na+-HCO −3 cotransport in basolateral membrane vesicles isolated from rabbit renal cortex. J. Clin. Invest. 83:345–351
Talor, Z., Richison, G., Arruda, J.A.L. 1985. High-affinity calcium binding sites in luminal and basolateral renal membranes. Am. J. Physiol. 248:F472-F478
Wright, E.M., Peerce, B.E. 1984. Identification and conformational changes of the intestinal proline carrier. J. Biol. Chem. 259:14993–14996
Wu, J.S.R., Lever, J.E. 1987. Monoclonal antibodies that bind the renal Na+/Glucose symport system. 2. Stabilization of an active conformation. Biochemistry 26:5790–5796
Author information
Authors and Affiliations
Additional information
The authors thank Carmen Hill and Clary Olichwier for excellent secretarial assistance in the preparation of this manuscript.
Rights and permissions
About this article
Cite this article
Stim, J., Bernardo, A.A., Kear, F.T. et al. Renal cortical basolateral Na+/HCO −3 cotransporter: II. Detection of conformational changes with fluorescein isothiocyanate labeling. J. Membarin Biol. 140, 39–46 (1994). https://doi.org/10.1007/BF00234484
Received:
Revised:
Issue Date:
DOI: https://doi.org/10.1007/BF00234484