Summary
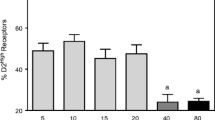
Dopamine (DA) D2 receptor binding is increased in the striatum of 5–6 months old weaver mutant mice (Kaseda et al. 1987). This may occur in response to the loss of DA neurons in the midbrain and the decrease in DA content in the striatum of homozygous mutants. One purpose of the present study was to determine if the diminished DA innervation is associated with changes in D2 receptors at earlier ages and if the increase in DA D2 receptor binding seen at 5–6 months is a lasting phenomenon. Specific [3H]spiperone binding was measured in the dorsolateral (DL), dorsomedial (DM) and ventrolateral (VL) striatum and in the nucleus accumbens (AC) of homozygous weaver mutant mice (wv/wv), heterozygous littermates (wv/+) and wild-type controls (+/+). Mice were studied at 20 days and 1, 3, 6, 9 and 12 months of age. The difference in specific [3H]spiperone binding in DL striatum between wv/wv and +/+ mice was significantly greater at 6 months than the difference at 1 month and at 12 months of age. Foetal ventral mesencephalic grafts survive and establish functional innervation in the striatum of weaver mice as shown by the induction of a contralateral turning bias (Low et al. 1987). The second aim of the present studies was to determine if such grafts would also reverse the increase in DA D2 receptor binding in the striatum. Aspiration cavities were prepared in the cortex of weaver mice, and ventral mesencephalic tissue from E14–E15 +/+ foetuses was subsequently placed on the surface of the right dorsal striatum when the recipients were 3 months old. At 6 months of age, specifie [3H]spiperone binding was determined in DM and DL striatum and in AC. Compared with the non-grafted side, the decreases in [3H]spiperone binding on the right (grafted) side in DL and DM striatum of the transplanted group were significantly greater than the differences in the other two groups. Since the grafts were introduced at an age (3 months) at which no significant difference in binding is seen between wv/wv and + / +, it appears that the grafts may prevent the increase in DA D2 receptor binding which is seen in non-grafted weaver mice at 6 months of age.
Similar content being viewed by others
References
Björklund A, Lindvall O, Isacson O, Brundin P, Wictorin K, Strecker RE, Clarke DJ, Dunnett SB (1987) Mechanisms of action of intracerebral neural implants: studies on nigral and striatal grafts to the lesioned striatum. Trends Neurosci 10:509–516
Björklund A, Stenevi U (1979) Reconstruction of the nigrostriatal dopamine pathway by intracerebral nigral transplants. Brain Res 177:555–560
Boyson SJ, McGonigle P, Molinoff PB (1986) Quantitative autoradiographic localization of the D1 and D2 subtypes of dopamine receptors in rat brain. J Neurosci 6:3177–3188
Broaddus WC, Bennett JP Jr (1990) Postnatal development of striatal dopamine function. II. Effects of neonatal 6-hydroxy-dopamine treatments on D1 and D2 receptors, adenylate cyclase activity and presynaptic dopamine function. Dev Brain Res 52:273–277
Brundin P, Strecker RE, Londos E, Björklund A (1987) Dopamine neurons grafted unilaterally to the nucleus accumbens affect drug-induced circling and locomotion. Exp Brain Res 69:183–194
Bugiani O, Perdelli F, Salvarani S, Leonardi A, Mancardi GL (1980) Loss of striatal neurons in Parkinson's disease: a cytometric study. Eur Neurol 19:339–344
Creese I, Snyder SH (1979) Nigrostriatal lesions enhance striatal 3H-apomorphine and 3H-spiroperidol binding. Eur J Pharmacol 56:277–281
Deskin R, Seidler FJ, Whitmore WL, Slotkin TA (1981) Development of α-noradrenergic and dopaminergic receptor systems depends on maturation of their presynaptic nerve terminals in the rat brain. J Neurochem 36:1683–1690
Doucet G, Brundin P, Seth S, Murata Y, Strecker RE, Triarhou LC, Ghetti B, Björklund A (1989) Degeneration and graft-induced restoration of dopamine innervation in the weaver mouse neostriatum: a quantitative radioautographic study of [3H]dopamine uptake. Exp Brain Res 77:552–568
Dunnett SB, Björklund A, Stenevi U, Iversen SD (1981) Behavioural recovery following transplantation of substantia nigra in rats subjected to 6-OHDA lesions of the nigrostriatal pathway. I. Unilateral lesions. Brain Res 215:147–161
Freed WJ, Ko GN, Niehoff DL, Kuhar MJ, Hoffer BJ, Olson L, Cannon-Spoor HE, Morihisa JM, Wyatt RJ (1983) Normalization of spiroperidol binding in the denervated rat striatum by homologous grafts of substantia nigra. Science 222:937–939
Freed WJ, Olson L, Ko GN, Morihisa JM, Niehoff D, Strömberg I, Kuhar M, Hoffer BJ, Wyatt RJ (1985) Intraventricular substantia nigra and adrenal medulla grafts: mechanisms of action and [3H]spiroperidol autoradiography. In: Björklund A, Stenevi U (eds) Neural grafting in the mammalian CNS. Elsevier Science Publishers BV, Amsterdam, pp 471–488
Freed WJ, Perlow MJ, Karoum F, Seiger Å, Olson L, Hoffer BJ, Wyatt RJ (1980) Restoration of dopaminergic function by grafting of fetal rat substantia nigra to the caudate nucleus: long-term behavioral, biochemical and histochemical studies. Ann Neurol 8:510–519
Freund TF, Bolam JP, Björklund A, Stenevi U, Dunnett SB, Powell JF, Smith AD (1985) Efferent synaptic connections of grafted dopaminergic neurons reinnervating the host neostriatum: a tyrosine hydroxylase immunocytochemical study. J Neurosci 5:603–616
Games PA (1977) An improved t-table for simultaneous control on g contrasts. J Am Stat Assoc 72:531–534
Gupta M, Felten DL, Ghetti B (1987) Selective loss of monoaminergic neurons in weaver mutant mice: an immunocytochemical study. Brain Res 402:379–382
Hattori T, Fibiger HC (1982) On the use of lesions of afferents to localize neurotransmitter receptor sites in the striatum. Brain Res 238:245–250
Heikkila RE, Shapiro BS, Duvoisin RC (1981) The relationship between loss of dopamine nerve terminals, striatal 3H-spiroperidol binding and rotational behavior in unilaterally 6-hydroxydopamine-lesioned rats. Brain Res 211:285–292
Kaseda Y, Ghetti B, Low WC, Richter JA, Simon JR (1987) Dopamine D2 receptors increase in the dorsolateral striatum of weaver mutant mice. Brain Res 422:178–181
Kelly PH, Moore KE (1976) Mesolimbic dopaminergic neurones in the rotational model of nigrostriatal function. Nature (Lond) 263:695–696
Lane JD, Nadi NS, McBride WJ, Aprison MH, Kusano K (1977)Contents of serotonin, norepinephrine and dopamine in the cerebrum of the “staggerer”, “weaver” and “nervous” neurologically mutant mice. J Neurochem 29:349–350
Lindvall O, Rehncrona S, Brundin P, Gustavii B, Åstedt B, Widner H, Lindholm T, Björklund A, Leenders KL, Rothwell JC, Frackowiak R, Marsden CD, Johnels B, Steg G, Freedman R, Hoffer BJ, Seiger Å, Bygdeman M, Strömberg I, Olson L (1989)Human fetal dopamine neurons grafted into the striatum in two patients with severe Parkinson's disease. A detailed account of methodology and a 6-month follow-up. Arch Neurol 46:615–631
Lindvall O, Brundin P, Widner H, Rehncrona S, Gustavii B, Frackowiak R, Leenders KL, Sawle G, Rothwell JC, Marsden CD, Björklund A (1990) Grafts of fetal dopamine neurons survive and improve motor function in Parkinson's disease. Science 247:574–577
List SJ, Seeman P (1981) Resolution of dopamine and serotonin receptor components of 3H-spiperone binding to rat brain regions. Proc Natl Acad Sci USA 78:2620–2624
Low WC, Triarhou LC, Kaseda Y, Norton J, Ghetti B (1987)Functional innervation of the striatum by ventral mesencephalic grafts in mice with inherited nigrostriatal dopamine deficiency. Brain Res 435:315–321
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with folin phenol reagent. J Biol Chem 193:265–275
Milliken GA, Johnson DE (1984) Analysis of messy data, Vol 1. Designed experiments. Van Nostrand Reinhold, New York
Munson PJ, Rodbard D (1980) LIGAND: a versatile computerized approach for characterization of ligand-binding systems. Anal Biochem 107:220–239
Neter J, Wasserman W, Kutner MH (1985) Applied linear statistical models, 2nd edn. Richard D. Irwin Inc., Homewood
Ohta K, Graybiel AM, Roffler-Tarlov S (1989) Dopamine D1 binding sites in the striatum of the mutant mouse weaver. Neurosci 28:69–82
Oppenheimer DR (1984) Diseases of the basal ganglia, cerebellum and motor neurons. In: Hume JH, Corsellis JAN, Duchen LW (eds) Greenfield's neuropathology. Wiley, New York, pp 699–747
Perlow MJ, Freed WJ, Hoffer BJ, Seiger Ă, Olson L, Wyatt RJ (1979) Brain grafts reduce motor abnormalities produced by destruction of nigrostriatal dopamine system. Science 204:643–647
Rakic P, Sidman RL (1973) Sequence of developmental abnormalities leading to granule cell deficit in cerebellar cortex of weaver mutant mice. J Comp Neurol 152:103–132
Roffler-Tarlov S, Graybiel AM (1984) Weaver mutation has differential effects on the dopamine-containing innervation of the limbic and nonlimbic striatum. Nature (Lond) 307:62–66
Roffler-Tarlov S, Graybiel AM (1986) Expression of the weaver gene in dopamine-containing neural systems is dose-dependent and affects both striatal and nonstriatal regions. J Neurosci 6:3319–3330
Roffler-Tarlov S, Graybiel AM (1987) The postnatal development of the dopamine-containing innervation of dorsal and ventral striatum: effects of the weaver gene. J Neurosci 7:2364–2372
Roffler-Tarlov S, Pugatch D, Graybiel AM (1990) Patterns of cell and fiber vulnerability in the mesostriatal system of the mutant mouse weaver. II. High affinity uptake sites for dopamine. J Neurosci 10:734–740
Rose G, Gerhardt G, Strömberg I, Olson L, Hoffer B (1985) Monoamine release from dopamine-depleted rat caudate nucleus reinnervated by substantia nigra transplants: an in vivo electrochemical study. Brain Res 341:92–100
Schmidt MJ, Sawyer BD, Perry KW, Fuller RW, Foreman MM, Ghetti B (1982) Dopamine deficiency in the weaver mutant mouse. J Neurosci 2:376–380
Sidman RL, Green MC, Appel SH (1965) Catalog of the neurological mutants of the mouse. Harvard University Press, Cambridge, pp 66–67
Sonsalla PK, Manzino L, Heikkila RE (1988) Interactions of D1 and D2 dopamine receptors on the ipsilateral vs contralateral side in rats with unilateral lesions of the dopaminergic nigrostriatal pathway. J Pharmacol Exp Ther 247:180–185
Strecker RE, Sharp T, Brundin P, Zetterström T, Ungerstedt U, Björklund A (1987) Autoregulation of dopamine release and metabolism by intrastriatal nigral grafts as revealed by intracerebral dialysis. Neuroscience 22:169–178
Triarhou LC, Brundin P, Doucet G, Norton J, Björklund A, Ghetti B (1990) Intrastriatal implants of mesencephalic cell suspensions in weaver mutant mice: ultrastructural relationships of dopaminergic dendrites and axons issued from the graft. Exp Brain Res 79:3–17
Triarhou LC, Low WC, Ghetti B (1986) Transplantation of ventral mesencephalic anlagen to hosts with genetic nigrostriatal dopamine deficiency. Proc Natl Acad Sci USA 83:8789–8793
Triarhou LC, Low WC, Ghetti B (1987) Synaptic investment of striatal cellular domains by grafted dopamine neurons in weaver mutant mice. Naturwissenschaften 74:591–593
Triarhou LC, Low WC, Norton J, Ghetti B (1988a) Reinstatement of synaptic connectivity in the striatum of weaver mutant mice following transplantation of ventral mesencephalic anlagen. J Neurocytol 17:233–243
Triarhou LC, Norton J, Ghetti B (1988b) Mesencephalic dopamine cell deficit involves areas A8, A9 and A10 in weaver mutant mice. Exp Brain Res 70:256–265
Triarhou LC, Norton J, Ghetti B (1988c) Synaptic connectivity of tyrosine hydroxylase immunoreactive nerve terminals in the striatum of normal, heterozygous and homozygous weaver mutant mice. J Neurocytol 17:221–232
Winer BJ (1971) Statistical principles in experimental design, 2nd edn. McGraw-Hill, New York
Zahniser NR, Dubocovich ML (1983) Comparison of dopamine receptor sites labeled by [3H]-S-sulpiride and [3H]-spiperone in striatum. J Pharmacol Exp Ther 227:592–599
Zetterström T, Brundin P, Gage FH, Sharp T, Isacson O, Dunnett SB, Ungerstedt U, Björklund A (1986) In vivo measurement of spontaneous release and metabolism of dopamine from intrastriatal nigral grafts using intracerebral dialysis. Brain Res 362:344–349
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Kaseda, Y., Ghetti, B., Low, W.C. et al. Age-related changes in striatal dopamine D2 receptor binding in weaver mice and effects of ventral mesencephalic grafts. Exp Brain Res 83, 1–8 (1990). https://doi.org/10.1007/BF00232187
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00232187