Summary
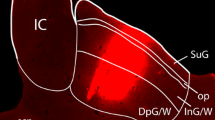
Immunocytochemistry for serotonin (5-HT) was carried out in both hamsters and rats in order to determine whether or not 5-HT-positive cells existed in the superior colliculus (SC) of either species. In both hamster and rat, the superficial and deep SC laminae contained dense networks of 5-HT-positive fibers. The rat's SC contained no 5-HT-positive neurons. In hamster, numerous 5-HT-immunoreactive cells were visible throughout the depth of the stratum griseum superficiale (SGS). These neurons had a variety of morphological characteristics and included marginal cells, horizontal cells, and neurons with vertically oriented dendritic trees. No 5-HT-positive neurons were found in any other portion of the hamster's SC. 5-HT-positive SC cells were observed with antisera from two different sources and they were not seen in animals that were pretreated with reserpine. Pretreatment with fluoxetine (an inhibitor of 5-HT uptake) also resulted in a disappearance of 5-HT-positive neurons in the hamster's SC. This result indicated that “serotonergic” cells in the colliculus of this species are capable of taking up, but probably not synthesizing, this indoleamine. The dorsal and ventral lateral geniculate nuclei (LGNd and LGNv, respectively) both contain numerous 5-HT-positive fibers and both of these structures receive input from the SGS. Combination of retrograde tracing with fluorogold and immunocytochemistry indicated that 5-HT-accumulating SC neurons were not the source of these fibers. Unilateral ablation of the superficial SC laminae also failed to reduce 5-HT immunoreactivity in either the LGNd or LGNv. These results are consistent with the possibility that 5-HT-accumulating cells in the hamster's SC may be interneurons that take up this transmitter after it is released by afferents to this nucleus.
Similar content being viewed by others
References
Abramson BP, Chalupa LM (1988) Multiple pathways from the superior colliculus to the extra-geniculate visual thalamus of the cat. J Comp Neurol 271:397–418
Ahlsen G (1984) Brain stem neurones with differential projection to functional subregions of the dorsal lateral geniculate complex in the cat. Neuroscience 12:817–838
Ahlsen G, Lo FS (1982) Projection of brain stem neurons to the perigeniculate nucleus and the lateral geniculate nucleus in the cat. Brain Res 238:433–438
Bennett-Clarke C, Mooney RD, Chiaia NL, Rhoades RW (1989) A substance P projection from the superior colliculus to the parabigeminal nucleus in the rat and hamster. Brain Res 500:1–11
Bobillier P, Petitjean F, Salvert D, Ligier M, Seguin S (1975) Differential projections of the nucleus raphe dorsalis and nucleus raphe centralis as revealed by autoradiography. Brain Res 85:205–210
Brunken WJ, Daw NW (1987) The actions of serotonergic agonists and antagonists on the activity of brisk ganglion cells in the rabbit retina. J Neurosci 7:4054–4065
Chalupa LM, Rhoades RW (1977) Responses of visual, somatosensory and auditory neurons in the golden hamster's superior colliculus. J Physiol (Lond) 270:595–626
Chang J-Y, Ekblad E, Kannisto P, Owman C (1989) Serotonin uptake into cerebrovascular nerve fibers of rat, visualization by immunocytochemistry, disappearance following sympathectomy, and release during electrical stimulation. Brain Res 492:79–88
Conrad LCA, Leonard CM, Pfaff DW (1974) Connections of median and dorsal raphe nuclei in the rat: an autoradiographic and degeneration study. J Comp Neurol 156:179–206
Consolazione A, Milstein C, Wright B, Cuello AC (1981) Immunocytochemical detection of serotonin with monoclonal antibodies. J Histochem Cytochem 29:1425–1430
Cropper EC, Eisenman JS, Azmitia EC (1984) An immunocytochemical study of the serotonergic innervation of the thalamus of the rat. J Comp Neurol 224:38–50
Cudennec A, Duverger D, Nishikawa T, McRae-Degueurce A, MacKenzie ET, Scatton B (1988) Influence of ascending serotonergic pathways on glucose use in the conscious rat brain. I. Effects of electrolytic or neurotoxic lesions of the dorsal and/or medial raphe nucleus. Brain Res 444:214–226
DeLima AD, Singer W (1987) The serotonergic fibers in the dorsal lateral geniculate nucleus of the cat: distribution and synaptic connections demonstrated with immunocytochemistry. J Comp Neurol 258:339–351
Dowling JE, Ehinger B, Floren I (1980) Fluorescence and electron microscopical observations on the amine-accumulating neurons of the cebus monkey retina. J Comp Neurol 192:665–685
Ehinger B, Floren I (1976) Indoleamine-accumulating neurons in the retina of the rabbit, cat and goldfish. Cell Tiss Res 175:37–48
Ehinger B, Floren I, Tornqvist K (1982) Autoradiography of (3H)-5-hydroxytryptamine uptake in the retina of some mammals. Graefes Arch Klin Exp Opthalmol 218:1–8
Elde R, Hökfelt T, Johansson O, Terenius L (1976) Immunohistochemical studies using antibodies to leucineenkephalin: initial observations on the nervous system of the rat. Neuroscience 1:349–351
Finley JCW, Maderdrut JL, Petrusz P (1981) The immunocytochemical localization of enkephalin in the central nervous system of the rat. J Comp Neurol 198:541–565
Fukuda Y, Iwama K (1978) Visual receptive-field properties of single cells in the rat superior colliculus. Jpn J Physiol 28:385–400
Graham J, Casagrande VA (1980) A light microscopic and electron microscopic study of the superficial layers of the superior colliculus of the tree shrew (Tupaia glis). J Comp Neurol 191:133–151
Graybiel AM, Brecha N, Karten HJ (1984) Cluster-and-sheet pattern of enkephalin-like immunoreactivity in the superior colliculus of the cat. Neuroscience 12:191–214
Hsu SM, Raine L, Farger H (1981) Use of avidin-biotin peroxidase complex (ABC) in immunoperoxidase techniques: a comparison between ABC and unlabeled antibody (PAP) procedures. J Histochem Biochem 29:577–580
Huerta MF, Harting JK (1983) Sublamination within the superficial gray layer of the squirrel monkey: an analysis of the tectopulvinar projection using anterograde and retrograde transport methods. Brain Res 261:119–126
Huerta MF, Harting JK (1984) The mammalian superior colliculus: studies of its morphology and connections. In: Vanegas H (ed) comparative neurology of the optic tectum. Plenum Publishing, New York, pp 687–773
Humphrey NK (1968) Responses to visual stimuli of units in the superior colliculus of rats and monkeys. Exp Neurol 20:312–340
Kanaseki T, Sprague JM (1974) Anatomical organization of pretectal nuclei and tectal laminae in the cat. J Comp Neurol 158:319–338
Kawamura S, Kobayashi E (1975) Identification of laminar origin of some tectothalamic fibers in the cat. Brain Res 91:281–285
Kemp JA, Roberts HC, Sillito AM (1982) Further studies on the action of 5-hydroxytryptamine in the lateral geniculate nucleus of the cat. Brain Res 146:334–337
Langer TP, Lund RD (1974) The upper-layers of the superior colliculus of the rat: a Golgi study. J Comp Neurol 158:405–436
Lewis ME, Khachaturian H, Akil H, Watson SJ (1984) Anatomical relationship between opioid peptides and receptors in rhesus monkey brain. Brain Res Bull 13:801–812
Lugo-Garcia N, Kicliter E (1987) Superior colliculus efferents to five subcortical visual system structures in the ground squirrel. Brain Res 426:131–141
Mackay-Sim A, Sefton AJ, Martin PR (1983) Subcortical projections to lateral geniculate and thalamic reticular nuclei in the hooded rat. J Comp Neurol 213:24–35
Marc RE, Liu W-S, Scholz K, Muller JF (1988) Serotonergic and serotonin-accumulating neurons in the goldfish retina. J Neurosci 8:3427–2450
Mathers LH (1977) Postnatal maturation of neurons in the rabbit superior colliculus. J Comp Neurol 173:439–456
Mize RR, Payne MP (1987) The innervation density of serotonergic (5-HT) fibers varies in different subdividions of the cat lateral geniculate nucleus complex. Neurosci Lett 82:133–139
Mooney RD, Klein BG, Rhoades RW (1985) Correlations between the structural and functional characteristics of neurons in the superficial laminae and the hamster's superior colliculus. J Neurosci 5:2989–3009
Mooney RD, Nikoletseas MM, Ruiz SA, Rhoades RW (1988) Receptivefield properties and morphological characteristics of the superior collicular neurons that project to the lateral posterior and dorsal lateral geniculate nuclei in the hamster. J Neurophysiol 59:1333–1351
Raczkowski D, Diamond IT (1981) Projections from the superior colliculus and the neocortex to the pulvinar nucleus in Galago. J Comp Neurol 200:231–254
Robson JA, Hall WC (1976) Projections from the superior colliculus to the dorsal lateral geniculate nucleus of the grey squirrel (Sciurus carolinensis). Brain Res 113:379–385
Robson JA, Hall WC (1977) The organization of the pulvinar in the grey squirrel (Sciurus carolinensis). J Comp Neurol 173:355–388
Rogawski MA, Aghajanian GK (1980) Norepinephrine and serotonin: opposite effects on the activity of lateral geniculate neurons evoked by optic pathway stimulation. Exp Neurol 69:678–694
Sandell JH, Masland RH (1986) A system of indoleaminea-ccumulating neurons in the rabbit retina. J Neurosci 6:3331–3347
Senba E, Daddona PE, Nagy JI (1987) Development of adenosine deaminase-immunoreactive neurons in the rat brain. Dev Brain Res 31:59–71
Smolen AJ, Wright LL, Cunningham TJ (1983) Neuron numbers in the superior cervical sympathetic ganglion of the rat: a critical comparison of methods for cell counting. J Neurocytol 12:739–750
Steinbusch, HWM (1984) Serotonin-immunoreactive neurons and their projections in the CNS. In: Björklund A, Hökfelt T, Kuhar MJ (eds) Handbook of chemical neuroanatomy, Vol 3. Classical transmitters and transmitter receptors in the CNS, Part II. Elsevier Science Publishers, Amsterdam, pp 68–125
Steinbusch HWM, Verhofstad AAJ, Joosten HWJ (1978) Localization of serotonin in the central nervous system by immunohistochemistry: description of a specific and sensitive technique and some applications. Neuroscience 3:811–819
Steinbusch HWM (1981) Distribution of serotonin-immunoreactivity in the central nervous system of the rat cell bodies and terminals. Neuroscience 6:557–618
Sterling P (1971) Receptive fields and synaptic organization of the superficial gray layer of the cat superior colliculus. Vis Res Suppl 3:309–328
Sugita S, Otani K, Tokunaga A, Terasawa K (1983) Laminar origin of the tectothalamic projections in the albino rat. Neurosci Lett 43:143–147
Takeuchi Y, Kimura H, Sano Y (1982) Immunohistochemical demonstration of the distribution of serotonin neurons in the brainstem of the rat and cat. Cell Tiss Res 224:247–267
Taylor AM, Jeffery G, Lieberman AR (1986) Subcortical afferent and efferent connections of the superior colliculus in the rat and comparisons between albino and pigmented strains. Exp Brain Res 62:131–142
Tebecis AK, Di Maria A (1972) A re-evaluation of the mode of action of 5-hydroxytryptamine on lateral geniculate neurones: comparison with catecholamines and LSD. Exp Brain Res 14:480–493
Thier P, Wassle H (1984) Indoleamine-mediated reciprocal modulation of on-centre and off-centre ganglion cell activity in the retina of the cat. J Physiol (Lond) 351:613–630
Tokunaga A, Otani K (1976) Dendritic patterns of neurons of rat superior colliculus. Exp Neurol 52: 189–205
Ugrumov MV, Taxi J, Steinbusch HWM, Tramu G, Mitskevich MS (1989) On the distribution and morpho-functional characteristics of 5-HT-immunoreactive cells in the hypothalamus of fetuses and neonatal rats. Dev Brain Res 46:233–241
Valverde F (1973) The neuropil in superficial layers of the superior colliculus of the mouse. Anat Entwickl-Gesch 142:117–147
Vaney DI (1986) Morphological identification of serotoninaccumulating neurons in the living retina. Science 233:444–446
Wilson JR, Hendrickson AE (1988) Serotonergic axons in the monkey's lateral geniculate nucleus. Vis Neurosci 1:125–133
Yamamoto C (1974) Electrical activity recorded from thin sections of the lateral geniculate body, and the effects of 5-hydroxytryptamine. Exp Brain Res 19:271–281
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Bennett-Clarke, C.A., Mooney, R.D., Chiaia, N.L. et al. Serotonin immunoreactive neurons are present in the superficial layers of the hamster's, but not the rat's, superior colliculus. Exp Brain Res 85, 587–597 (1991). https://doi.org/10.1007/BF00231743
Received:
Revised:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00231743