Summary
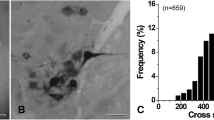
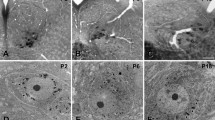
The differential distribution of glutamate (Glu), aspartate (Asp), glycine (Gly), gamma-aminobutyric acid (GABA) and taurine (Tau) was investigated in the cat's perihypoglossal nuclei. Serial semi-thin (0.5 μm) sections through the perihypoglossal nuclei were incubated with antisera raised against the mentioned amino acids with the aim of studying possible co-localization. In each experiment different measures were undertaken in order to screen for possible cross-reactivities, and all sections were processed together with test conjugates in order to ascertain the specificity of the antisera used. A very high proportion of the neurons in the perihypoglossal nuclei (about 90%) shows strong immunostaining for Asp and also displays distinct immunoreactivity for Glu in neighbouring sections. About 25% of the cells in the perihypoglossal nuclei are intensely immunostained for Gly, but very few cells show immunoreactivity for GABA. Only glial cells appear to be immunostained for Tau. Neurons that are Gly(+) also display Glu and Asp immunoreactivities. The neuropil of the perihypoglossal nuclei shows a high density of GABA(+), Gly(+) and Glu(+) puncta mainly representing stained axons and terminals. Fewer Asp(+) puncta and very few Tau(+) nerve terminal-like puncta are seen. Details of the regional distribution of immunopositive neurons and puncta within the perihypoglossal nuclei are described. The findings are discussed with particular reference to the possible role of the mentioned amino acids as transmitter substances in the known synaptic circuitry of the perihypoglossal nuclei.
Similar content being viewed by others
References
Angaut P, Brodal A (1979) The projection of the vestibulo-cerebellum onto the vestibular nuclei in the cat. Arch Ital Biol 105:441–479
Aoki E, Semba R, Kato K, Kashiwamata S (1987) Purification of specific antibody against aspartate and immunocytochemical localization of aspartergic neurons in the rat brain. Neuroscience 21:755–765
Baker R, Berthoz A (1975) Is the prepositus hypoglossi nucleus the source of another vestibulo-ocular pathway? Brain Res 86:121–127
Baker R, Gresty M, Berthoz A (1975) Neuronal activity in the prepositus hypoglossi nucleus correlated with vertical and horizontal eye movement in the cat. Brain Res 101:366–371
Baker R, Berthoz A, Delgado-Garcia J (1977) Monosynaptic excitation of trochlear motor neurons following electrical stimulation of the prepositus hypoglossi nucleus. Brain Res 121:157–161
Brodal A (1952) Experimental demonstration of cerebellar connections from the perihypoglossal nuclei (nucleus intercalatus, nucleus prepositus hypoglossi and nucleus of Roller) in the cat. J Anat 86:110–128
Brodal A (1983) The perihypoglossal nuclei in the macaque monkey and the chimpanzee. J Comp Neurol 218:257–269
Brodal A, Brodal P (1983) Observations on the projection from the perihypoglossal nuclei onto the cerebellum in the macaque monkey. Arch Ital Biol 121:151–166
Campistron G, Buijs RM, Geffard M (1986) Specific antibodies against aspartate and their immunocytochemical application in the rat brain. Brain Res 365:179–184
Carleton SC, Carpenter MB (1983) Afferent and efferent connections of the medial, inferior and lateral vestibular nuclei in the cat and monkey. Brain Res 278:29–51
Carpenter MB, Batton RR (1980) Abducens internuclear neurons in their role in conjugate horizontal gaze. J Comp Neurol 189:191–209
Cheron G, Gillis P, Godaux E (1986a) Lesions in the cat prepositus complex: effects on the vestibulo-ocular reflex and saccades. J Physiol 372:75–94
Cheron G, Gillis P, Godaux E (1986b) Lesions in the cat prepositus complex: effects on the optokinetic system. J Physiol 372:95–111
Dale N, Ottersen OP, Roberts A, Storm-Mathisen J (1986) Inhibitory neurones of a motor pattern generator in Xenopus revealed by antibodies to glycine. Nature 234:255–257
Dememes D, Raymond J (1982) Radioautographic identification of (3H) glutamic acid labeled nerve endings in the cat oculomotor nucleus. Brain Res 231:433–437
Docherty M, Bradford HF, Wu J-Y (1987) Co-release of glutamate and aspartate from cholinergic and GABAergic synaptosomes. Nature 330:64–65
Fonnum F (1984) Glutamate: a neurotransmitter in mammalian brain. J Neurochem 46:1–11
Gacek RR (1977) Location of brain stem neurons projecting to the oculomotor nucleus in the cat. Exp Neurol 57:725–749
Gacek RR (1979) Location of abducens afferent neurons in the cat. Exp Neurol 64:342–353
Gould BB (1980) Organization of afferents from brain stem nuclei to the cerebellar cortex in the cat. Adv Anat Embryol Cell Biol 62:1–90
Graf W, McCrea RA, Baker R (1983) Morphology of posterior canal related secondary vestibular neurons in rabbit and cat. Exp Brain Res 52:125–138
Grantyn A, Grantyn R (1982) Axonal pattern and sites of termination of cat superior colliculus neurons projecting in the tecto-bulbospinal tract. Exp Brain Res 46:243–257
Graybiel AM (1977) Direct and indirect preoculomotor pathways of the brainstem: an autoradiographic study of the pontine reticular formation in the cat. J Comp Neurol 175:37–78
Graybiel AM, Hartwieg EA (1974) Some afferent connections of the oculomotor complex in the cat: an experimental study with tracer techniques. Brain Res 81:543–551
Gresty M, Baker R (1976) Neurons with visual receptive field, eye movement and neck displacement sensitivity within and around the nucleus prepositus hypoglossi in the alert cat. Brain Res 24:429–433
Hepler JR, Toomim CS, McCarthy KD, Conti F, Battaglia G, Rustioni A, Petrusz P (1988) Characterization of antisera to glutamate and aspartate. J Histochem Cytochem 36:13–22
Hikosaka O, Igusa Y, Imai H (1980) Inhibitory connections of nystagmus-related reticular burst neurons with neurons in the abducens, prepositus hypoglossi and vestibular nuclei in the cat. Exp Brain Res 39:301–312
Holstege G, Collewijn H (1982) The efferent connections of the nucleus of the optic tract and the superior colliculus in the rabbit. J Comp Neurol 209:135–175
Ishizuka N, Mannen J, Sasaki SI, Shimazu H (1980) Axonal branches and terminations in the cat abducens nucleus of secondary vestibular neurons in the horizontal canal system. Neurosc Letters 16:143–148
Kotchabhakdi N, Hoddevik GH, Walberg F (1978) Cerebellar afferent projections from the perihypoglossal nuclei: an experimental study with the method of retrograde axonal transport of horseradish peroxidase. Exp Brain Res 31:13–29
Kumoi K, Sato N, Tanaka C (1987) Immunohistochemical localization of γ-aminobutyric acid and aspartate-containing neurons in the guinea pig vestibular nuclei. Brain Res 416:22–33
Lopez-Barneo J, Darlot C, Berthoz A, Baker R (1982) Neuronal activity in prepositus nucleus correlated with eye movement in the alert cat. J Neurophysiol 47:329–352
Maciewicz RJ, Eagen K, Kaneko CRS, Highstein SM (1977) Vestibular and medullary brain stem afferents to the abducens nucleus in the cat. Brain Res 123:229–240
Madl JE, Beitz AJ, Johnson RL, Larson AA (1987) Monoclonal antibodies specific for fixative-modified aspartate: immunocytochemical localization in the rat CNS. J Neurosci 7(9): 2639–2650
Madsen S, Ottersen OP, Storm-Mathisen J (1985) Immunocytochemical visualization of taurine: neuronal localization in the rat cerebellum. Neurosci Lett 60:255–260
McCrea RA, Baker R (1985a) Cytology and intrinsic organization of the perihypoglossal nuclei in the cat. J Comp Neurol 237:360–376
McCrea RA, Baker R (1985b) Anatomical connections of the nucleus prepositus of the cat. J Comp Neurol 237:377–407
McCrea RA, Baker R, Delgado-Garcia J (1979) Afferent and efferent organization of the prepositus hypoglossi nucleus. In: Granit R, Popeiano O (eds) Reflex control of posture and movements. Progr Brain Res 50:653–665
Mehler WR, Feferman ME, Nauta WJH (1960) Ascending axon degeneration following anterolateral cordotomy: an experimental study in the monkey. Brain 83:718–750
Mergner T, Pompeiano O, Corvaja N (1977) Vestibular projections to the nucleus intercalatus of Staderini mapped by retrograde transport of horseradish peroxidase. Neurosci Lett 5:303–313
Ottersen OP (1987) Postembedding light- and electron microscopic immunocytochemistry of amino acids: description of a new model system allowing identical conditions for specificity testing and tissue processing. Exp Brain Res 69:167–174
Ottersen OP, Storm-Mathisen J (1984a) Glutamate- and GABA-containing neurons in the mouse and rat brain, as demonstrated with a new immunocytochemical technique. J Comp Neurol 229:374–392
Ottersen OP, Storm-Mathisen J (1984b) Neurones containing or accumullating transmitter amino acids. In: Björklund A, Hökfelt T, Kuhar MJ (eds) Handbook of chemical neuroanatomy. Elsevier, Amsterdam, pp 141–246
Ottersen OP, Storm-Mathisen J (1985) Different neuronal localization of aspartate-like and glutamate-like immunoreactivities in the hippocampus of rat, guinea pig and Senegalese baboon, Papio papio, with a note on the distribution of γ-aminobutyrate. Neuroscience 16:589–606
Ottersen OP Storm-Mathisen J (1987) Distribution of inhibitory amino acid neurons in the cerebellum with some observations on the spinal cord: an immunocytochemical study with antisera against fixed GABA, glycine, taurine and β-alanine. J Mind Behav 8:503–518
Ottersen OP, Bramham CR (1988) Quantitative electron microscopic immunocytochemistry of excitatory amino acids. In: Cavalheiro EA, Lehmann J, Turski L (eds) Frontiers in excitatory amino acid research. Alan R Liss, New York, pp 93–100
Ottersen OP, Storm-Mathisen J, Somogyi P (1988) Colocalization of glycine-like and GABA-like immunoreactivities in Golgi cell terminals in the rat cerebellum: a postembedding light and electron microscopic study. Brain Res 450:342–353
Rinvik E, Ottersen OP, Storm-Mathisen J (1987) Gamma-aminobutyrate-like immunoreactivity in the thalamus of the cat. Neuroscience 21:781–805
Roffler-Tarlov S, Tarlov E (1975) Reduction of GABA synthesis following lesion of inhibitory vestibulotrochlear pathway. Brain Res 91:326–330
Sato Y, Kawasaki T, Ikarashi K (1982) Zonal organization of the floccular Purkinje cells projecting to the vestibular nucleus in cats. Brain Res 232:1–15
Somogyi P, Hodgson AJ, Smith AD, Nunzi MB, Gorio A, Wu J-Y (1984) Different populations of GABAergic neurons in the visual cortex and hippocampus of cat contain somatostatin- or cholecystokinin-immunoreactive material. J Neurosci 4:2590–2603
Somogyi P, Halasy K, Somogyi J, Storm-Mathisen J, Ottersen OP (1986) Quantification of immunogold labelling reveals enrichment of glutamate in mossy and parallel fibre terminals in cat cerebellum. Neuroscience 19:1045–1050
Steiger HJ, Buttner-Ennever JA (1979) Oculomotor nucleus afferents in the monkey demonstrated with horseradish peroxidase. Brain Res 160:1–15
Sternberger LA (1979) Immunocytochemistry, 2nd edn. Wiley, New York
Storm-Mathisen J, Leknes AK, Bore AT, Vaaland JL, Edmison P, Haug F-MS, Ottersen OP (1983) First visualization of glutamate and GABA in neurones by immunocytochemistry. Nature 301:517–520
Storm-Mathisen J, Ottersen OP (1983) Immunohistochemistry of glutamate and GABA. In: Hertz L, Kvamme E, McGeer EG, Shousboe A (eds) Glutamine, glutamate and GABA in the central nervous system. Alan R Liss, New York, pp 185–201
Storm-Mathisen J, Ottersen OP (1986) Antibodies against amino acid transmitters. In: Panula P, Paivarinta H, Soinila S (eds) Neurohistochemistry: modern methods and applications. Alan R Liss, New York, pp 107–136
Strassman A, Highstein SM, McCrea RA (1986a) Anatomy and physiology of saccadic burst neuron in the alert squirrel monkey. I. Excitatory burst neurons. J Comp Neurol 249:337–357
Strassman A, Highstein SM, McCrea RA (1986b) Anatomy and physiology of saccadic burst neurons in the squirrel monkey. II. Inhibitory burst neurons. J Comp Neurol 249:358–380
Walberg F, Dietrichs E, Nordby T (1985) On the projections from the vestibular and perihypoglossal nuclei to the spinal trigeminal and lateral reticular nuclei in the cat. Brain Res 333:123–130
Walberg F, Ottersen OP, Rinvik E (1990) GABA, glycine, aspartate, glutamate and taurine in the vestibular nuclei. An immunocytochemical investigation in the cat. Exp Brain Res (accepted for publication)
Weiss C, Disterholf JF (1985) Connections of the rabbit abducens nucleus. Brain Res 326:172–178
Yingcharoen K, Rinvik E (1983) Ultrastructural demonstration of a projection from the flocculus to the nucleus prepositus hypoglossi in the cat. Exp Brain Res 51:192–198
Author information
Authors and Affiliations
Additional information
Performed during leave of absence from Laboratory of Neurobiology, Center for Neuro-Behavioural Biology, Mahidol University, Salaya Campus, Bangkok, Thailand
Rights and permissions
About this article
Cite this article
Yingcharoen, K., Rinvik, E., Storm-Mathisen, J. et al. GABA, glycine, glutamate, aspartate and taurine in the perihypoglossal nuclei: an immunocytochemical investigation in the cat with particular reference to the issue of amino acid colocalization. Exp Brain Res 78, 345–357 (1989). https://doi.org/10.1007/BF00228906
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00228906