Summary
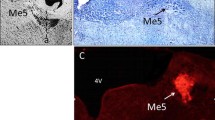
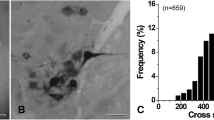
The central distributions of efferent and afferent components of the pharyngeal branches of the vagus (PH-X) and glossopharyngeal (PH-IX) nerves in the cat were studied by soaking their central cut ends in a horseradish peroxidase (HRP) solution. HRP-labelled PH-X neurones were distributed ipsilaterally in the rostral part of the nucleus ambiguus (NA) and the retrofacial nucleus (RFN); HRP-labelled PH-IX neurones were found in the ipsilateral RFN and the bulbopontine lateral reticular formation (RF). Vagal pharyngeal neurones constituted a large population of brainstem motoneurones. The population of HRP-labelled glossopharyngeal neurones was divided into two components. Indeed, on the basis of their location and somal morphology, the most ventral cells were identified as cranial motoneurones and those scattered in the lateral RF as parasympathetic preganglionic neurones. Application of HRP to the PH-IX nerve resulted also in the labelling of fibres and terminals in the alaminar spinal trigeminal nucleus and the nucleus of the solitary tract (NTS). The afferent fibres entered the lateral medulla with the glossopharyngeal roots, ran dorsomedially, then turned caudally toward the NTS and the caudal part of the alaminar spinal trigeminal motor (V) nucleus. In the NTS, labelled fibres ran mainly along the solitary tract, projecting to terminals in the dorsal and dorsolateral nuclei of the NTS.
Similar content being viewed by others
References
Anch AM, Remmers JE, Sauerland EK and Degroot WJ (1981) Oropharyngeal patency during waking and sleep in the Pick wickian syndrome: electromyographic activity of the tensor veli palatini. Electromyogr Clin Neurophysiol 21:317–330
Andrew BL (1956) A functional analysis of the myelinited fibres of the superior laryngeal nerve of the rat. J Physiol (Lond) 133:420–432
Barillot JC, Bianchi AL, Grélot L, Pio J, Roman J (1988) Le noyau ambigu chez le chat: représentation viscérotopique du pharynx. Arch Int Physiol Biochem 96:A185
Berman AL (1968) The brain stem of the cat: a cytoarchitectonic atlas with stereotaxic coordinates. University of Wisconsin Press, Madison
Bianchi AL, Grélot L, Iscoe S, Remmers JE (1988) Electrophysiological properties of rostral medullary respiratory neurones in the cat: an intracellular study. J Physiol (Lond) 407:293–310
Bieger D, Hopkins DA (1987) Viscerotopic representation of the upper alimentary tract in the medulla oblongata in the rat: the nucleus ambiguus. J Comp Neurol 262:546–562
Chibuzo GA, Cummings JF, Evans HE (1980) Autonomic innervation of the tongue: a horseradish peroxidase study in the dog. J Auton Nerv Syst 2:117–129
Contreras RJ, Gomez MM, Norgren R (1980) Central origins of cranial nerve parasympathetic neurons in the rat. J Comp Neurol 190:373–394
Davis PJ, Nail BS (1984) On the location and size of laryngeal motoneurons in the cat and rabbit. J Comp Neurol 230:13–32
Feindel W, Hinshaw JR, Weddell G (1952) The pattern of motor innervation in mammalian striated muscle. J. Anat 86:35–48
Guilleminault C, Hill MW, Simmons FB and Dement WC (1978) Obstuctive sleep apnea: electromyographic and fiberoptic studies. Exp Neurol 62:48–67
Grélot L, Bianchi AL, Iscoe S, and Remmers JE (1988) Expiratory neurones of the rostral medulla: anatomical and functional correlates. Neurosci Lett 89:140–145
Grélot L, Barillot JC, Bianchi AL (1989) Pharyngeal motoneurones: respiratory-related activity and responses to laryngeal afferents in the decerebrate cat. Exp Brain Res 78:336–344
Hamilton RB, Norgren R (1984) Central projections of gustatory nerves in the rat. J Comp Neurol 222:560–577
Hudson LC (1986) The origins of innervation of the canine caudal pharyngeal muscles: an HRP study. Brain Res 374:413–418
Hwang K, Grossman MI, Ivy AC (1948) Nervous control of the cervical portion of the oesophagus. Am J Physiol 154:343–357
Issa FG and Remmers JE (1989) Pathophysiology and treatment of obstructive sleep apnea. In: DH Simmon (ed) Current pulmonology. Year Book Medical Publishers Inc, Chicago, 10:327–352
Kalia M, Mesulam MM (1980) Brain stem projections of sensory and motor components of the vagus complex in the cat. II. Laryngeal, tracheobronchial, pulmonary, cardiac, and gastrointestinal branches. J Comp Neurol 193:467–508
Kalia M (1981) Anatomical organisation of the central respiratory neurons. Ann Rev Physiol 43:105–120
Lawn AM (1966) The localization in the nucleus ambiguus of the rabbit of the cells of origin of motor nerve fibers in the glossopharyngeal nerve and various branches of the vaguus nerve by means of retrograde degeneration. J Comp Neurol 127:293–305
Mesulam MM (1978) Tetramethyl benzidine for horseradish peroxidase neurohistochemistry: a non carcinogenic blue reaction-product with superior sensitivity for visualizing neural afferents and efferents. J Histochem Cytochem 26:106–117
Nomura S, Mizuno N (1982) Central distribution of afferent and efferent components of the glossopharyngeal nerve: an HRP study in the cat. Brain Res 236:1–13
Nonidez JF (1931) Innervation of the thyroid gland. II. Origin and course of the thyroid nerves in the dog. Am J Anat 57:135–169
Sinclair WJ (1971) Role of the pharyngeal plexus in initiation of swallowing. Amer J Physiol 221:1260–1263
Stuesse SL, Cruce WLR, Powell KS (1984) Organization within the cranial IX–X complex in ranid frogs: a horseradish peroxidase transport study. J Comp Neurol 222:358–365
Takana Y, Yoshida Y, Hirano M, Morimoto M, Kanaseki T (1987) Intramucosal distribution of the glossopharyngeal sensory fibers of cats. Brain Res Bull 19:115–127
Van Loveren H, Saunders MC, Cassini P, Keller JT (1985) Localization of motoneurons innervating the stylopharyngeus muscle in the cat. Neurosci Lett 58:251–255
Yoshida Y, Miyazaki T, Hirano M, Shin T, Totoki T, Kanaseki T (1980) Location of motoneurons supplying the cricopharyngeal muscle in the cat studied by means of the horseradish peroxidase method. Neurosci Lett 18: 1–4
Yoshida Y, Miyazaki T, Hirano M, Shin T, Totoki T, Kanaseki T (1981) Localization of efferent neurons innervating the pharyngeal constrictor muscles and the cervical esophagus muscle in the cat by means of the horseradish peroxidase method. Neurosci Lett 22:91–95
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Grélot, L., Barillot, J.C. & Bianchi, A.L. Central distributions of the efferent and afferent components of the pharyngeal branches of the vagus and glossopharyngeal nerves: an HRP study in the cat. Exp Brain Res 78, 327–335 (1989). https://doi.org/10.1007/BF00228904
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00228904