Abstract
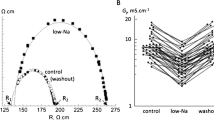
Short term (15 min) effects of activators of protein kinase A (PKA), PKC and PKG on cardiac macroscopic (gj) and single channel (γj) gap junctional conductances were studied in pairs of neonatal rat cardiomyocytes. Under dual whole-cell voltage-clamp, PKC activation by 100 nM TPA increased gj by 16 ± 2% (mean ± S.E.M, n=9), 1.5 mM of the PKG activator 8-bromo-cGMP (8Br-cGMP) decreased gj by 26 ± 2% (n=4), whereas 1.5 mM of the PKA activator 8Br-cAMP did not affect gj (1 ± 5%, n=11). Single cardiac gap junction channel events, resolved in the presence of heptanol, indicated two γj sizes of 20 pS and 40–45 pS. Under control conditions, the larger events were most frequently observed. Whereas 8Br-cAMP did not change this distribution, TPA or 8Br-cGMP shifted the γj distribution to the lower sizes. Diffusion of 6-carboxyfluorescein (6-CF), a gap junction permeant tracer, from the injected cell to neighboring cells was studied on small clusters of neonatal rat cardiomyocytes. Under control conditions, 6-CF labeled 8.4 ± 0.4 cells (mean ± S.E.M, n=31). Whereas 8Br-cAMP did not change the extent of dye transfer (8.1 ± 0.5 cells, n=10), TPA restricted the diffusion of 6-CF to 2.2 ± 0.2 cells (n=30) and 8Br-cGMP to 3.5 ± 0.3 cells (n=10). This suggests that permeability and single channel conductance of Cx43 gap junction channels are parallel related. Altogether, these results point to the differential modulation of electrical and metabolic coupling of cardiac cells by various phosphorylating conditions.
Similar content being viewed by others
References
Loewenstein WR: Junctional intercellular communication: the cell-to-cell membrane channel. Physiol Rev 61: 829–913, 1981
Barr L, Dewey MM, Berger W: Propagation of action potentials and the structure of the nexus in cardiac muscle. J Gen Physiol 48: 797–823, 1965
Gilula NB, Reeves OR, Steinbach A: Metabolic coupling, ionic coupling, and cell contacts. Nature 235: 262–265, 1972
Makowski L, Caspar DLD, Phillips WC, Goodenough DA: Gap junction structures. II. Analysis of the X-ray diffraction data. J Cell Biol 74: 629–645, 1977
Beyer EC, Paul DL, Goodenough DA: Connexin family of gap junction proteins. J Membr Biol 116: 187–194, 1990
Willecke K, Hennemann H, Dahl E, Jungbluth S, Heynkes R: The diversity of connexin genes encoding gap junctional proteins. Eur J Cell Biol 56: 1–7, 1991
Beyer EC, Paul DL, Goodenough DA: Connexin43: a protein from rat heart homologous to a gap junction protein from liver. J Cell Biol 105: 2621–2629, 1987
Beyer EC, Kistler J, Paul DL, Goodenough DA: Antisera directed against Connexin43 react with a 43-kD protein localized to gap junctions in myocardium and other tissues. J Cell Biol 108: 595–605, 1989
Kadle R, Zhang JT, Nicholson BJ: Tissue-specific distribution of differentially phosphorylated forms of Cx43. Mol Cell Biol 11: 363–369, 1991
Kanter HL, Saffitz JE, Beyer EC: Cardiac myocytes express multiple gap junction proteins. Circ Res 70: 438–444, 1992
Bastide B, Neyses L, Ganten D, Paul M, Willecke K, Traub O: Gap junction protein connexin40 is preferentially expressed in vascular endothelium and conductive bundels of rat myocardium and is increased under hypertensive conditions. Cite Res 73: 1138–1149, 1993
De Maziere A, Analbers L, Jongsma HJ, Gros D: Immunoelectron microscopic visualization of the gap junction protein connexin40 in the mammalian heart. Eur J Morph 30: 305–308, 1993
Gros D, Jarry-Guichard T, Ten Velde I, De Maziere A, van Kempen MJA, Davoust J, Briand JP, Moorman AFM, Jongsma HJ: Restricted distribution of connexin40, a gap junctional protein, in mammalian heart. Circ Res 74: 839–851, 1994
Musil LS, Beyer EC, Goodenough DA: Expression of the gap junction protein Connexin43 in embryonic chick lens: molecular cloning, ultrastructural localization, and post-translational phosphorylation. J Membr Biol 116: 163–175, 1990a
Musil LS, Cunningham BA, Edelman GM, Goodenough DA: Differential phosphorylation of the gap junction protein Connexin43 in junctional communication-competent and-deficient cell lines. J Cell Biol 111: 2077–2088, 1990b
Saez JC, Berthoud VM, Moreno AP, Spray DC: Gap junctions. Multiplicity of controls in differentiated and undifferentiated cells and possible fuctional implications. In: S. Shenolikar and A.C. Naim (eds). Advances in Second Messenger and Phosphoprotein Research, Vol. 27. Raven Press, New York, 1993, pp 163–198
De Bruijne J, Jongsma HJ: Membrane properties of aggregates of collagenase-dissociated rat heart cells. In: M. Tajuddin, P.K. Das, M. Tariq and N.S. Dhalla (eds). Advances in Myocardiology. University Park Press, Baltimore, 1980, pp 231–243
Neyton J, Trautmann A: Single-channel currents of an intercellular junction. Nature 317: 331–335, 1985
Takens-Kwak BR, Jongsma HJ, Rook MB, van Ginneken ACG: Mechanism of heptanol-induced uncoupling of cardiac gap junctions: a perforated patch-clamp study. Am J Physiol 262: C1531-C1538, 1992
Burt JM, Spray DC: Inotropic agents moduiate gap junctional conductance between cardiac myocytes. Am J Physiol 254: H1206-H1210, 1988
De Mello WC: Increase in junctional conductance caused by isoproterenol in heart cell pairs is suppressed by cAMP-dependent protein-kinase inhibitor. Biochem Biophys Res Comm 154: 509–514, 1988
Kwak BR, Hermans MMP, De Jonge HR, Lohmann SM, Jongsma HJ, Chanson M: Differential regulation of distinct types of gap junction channels by similar phosphorylating conditions. Mol Biol Cell 6: 1707–1719, 1995
Spray DC, Burt JM: Structure-activity relations of the cardiac gap junction channel. Am J Physiol 258: C195-C205, 1990
Münster PN, Weingart R: Effects of phorbol ester on gap junctions of neonatal rat heart cells. Pflügers Arch 423: 181–188, 1993
Bastide B, Hervé JC, Deleze J: The uncoupling effect of diacylglycerol on gap junctional communication of mammalian heart cells is independent of protein kinase C. Exp Cell Res 214: 519–527, 1994
Kikkawa U, Kishimoto A, Nishizuka Y: The protein kinase C family: heterogeneity and its implications. Annu Rev Biochem 58: 31–44, 1989
Lamers JMJ, Eskildsen-Helmond YEG, Resink AM, de Jonge HW, Bezstarosti K, Sharma HS, van Heugten HAA: Endothelin-1 induced phospholipase C-β and D and protein kinase c-isoenzyme signalling leading to hypertrophy in rat cardiomyocytes. J Cardiovasc Pharmacol, in press
Saez JC, Nairn AC, Czernic AJ, Spray DC, Hertzberg EL: Rat connexin43: regulation by phosphorylation in heart. In: J.E. Hall, G.A. Zampighi and R.M. Davis (eds). Progress in Cell Research, Vol.3. Elsevier, Amsterdam, 1993, pp 275–281
Takens-Kwak BR, Jongsma HJ: Cardiac gap junctions: three distinct single channel conductances and their modulation by phosphorylating treatments. Pflügers Arch 422: 198–200, 1992
Kwak BR, Saez JC, Wilders R, Chanson M, Fishman GI, Hertzberg EL, Spray DC, Jongsma HJ: Effects of cGMP-dependent phosphorylation on rat and human connexin43 gap junction channels. Pflügers Arch 430: 770–778, 1995
Veenstra RD, Wang H-Z, Westphale EM, Beyer EC: Multiple connexins confer distinct regulatory and conductance properties of gap junctions in developing heart. Circ Res 71: 1277–1283, 1992
Moreno AP, Saez JC, Fishman GI, Spray DC: Human connexin43 gap junction channels. Regulation of unitary conductances by phosphorylation. Circ Res 74: 1050–1057, 1994
Kennelly PJ, Krebs EG: Consensus sequences as substrate specificity determinants for protein kinases and protein phosphatases. J Biol Chem 266: 15555–15558, 1991
Azzi A, Boscoboinik D, Hensey C: The protein kinase C family Eur J Biochem 208: 547–557, 1992
Kemp BE, Pearson RB: Protein kinase recognition sequence motifs. Trends Biochem Sci 15: 342–346, 1990
Fishman GI, Spray DC, Leinwand LA: Molecular characterization and functional expression of the human cardiac gap junction channel. J Cell Biol 111: 589–598, 1990
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Kwak, B.R., Jongsma, H.J. Regulation of cardiac gap junction channel permeability and conductance by several phosphorylating conditions. Mol Cell Biochem 157, 93–99 (1996). https://doi.org/10.1007/BF00227885
Issue Date:
DOI: https://doi.org/10.1007/BF00227885