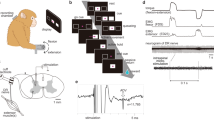
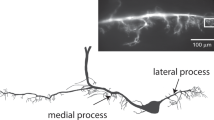
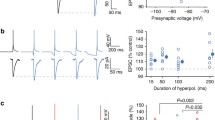
Conclusion
Presynaptic inhibition is a widespread mechanism among invertebrates and its study has developed quite independently from that which has occurred in vertebrates. However, it is striking that recent studies on presynaptic inhibition in sensory afferents of Arthropod have shown great similarities with presynaptic control exerted in the mammalian spinal cord primary afferents (Gossard et al. 1989; Rudomin et al. 1991): in both cases, presynaptic inhibition of sensory terminals involves inhibitory PADs associated with GABA, operating via Cl- ion channels. Sensory modulation exerted in this way may affect the reflex gain. Paired recordings on invertebrate preparations have made it possible to explain some of these mechanisms in greater detail, as was the case in lamprey (Alford et al. 1991).
Presynaptic mechanisms and particularly presynaptic inhibition have been studied mainly in neurons where the spike-initiating zone (initial segment of cell body in classic vertebrate neuron or the equivalent in the neurite of invertebrate neuron) is far from the output synapse. In such neurons spikes are actively conveyed in axons before they reach the output synapse. This is the case for primary afferents and for descending INs. In such cases presynaptic inhibition exerts a clear-cut effect onto spike-triggered synaptic transmission. However, a given axon generally divides into several branches, and therefore presynaptic inhibition activity onto some branches may not affect others. This possibility is likely to exist in local INs and is important to consider, in view of our understanding of how neuronal networks achieve their observed behavior. In the past, such networks were considered as input-output reflex chains; more recently, active properties that are essential for pattern generation (i.e., voltage-dependent and calcium-dependent conductances responsible for nonlinear properties of the membrane potential such as pacemaker potential and plateau properties), have been shown to exist in the component neurons; even more recently, connectivity as well as active properties on neurons have been demonstrated to be controlled by INs that could de novo rebuild new networks with specific functional role (Meyrand et al. 1994); the time has now come to consider that each neuron is not behaving as a single unit, but is rather made up of different compartments subjected to different local controls and local active properties (Coleman and Nusbaum 1994). This idea has already been supported by the demonstration that some neurons had several spike-initiating zones; it is reinforced by the finding that target cells are capable of presynaptically modulating their input fibers (Nusbaum et al. 1992). These findings seriously complicate the functional schema we had drawn of neuronal networks. However, this is certainly one of the most important challenges for the next decade, in view of understanding how the brain works.
Similar content being viewed by others
References
Alford S, Christenson J, Grillner S (1991) Presynaptic GABAA and GABAB receptor-mediated phasic modulation in axons of spinal motor interneurons. Eur J Neurosci 3:107–117
Alvarez-Leefmans FJ, Gamino SM, Giraldez F, Nogueron R (1988) Intracellular chloride regulation in amphibian dorsal root ganglion neurones studied with ionselective microelectrodes. J Physiol (Lond) 406:225–246
Atwood HL, Morin WA (1970) Neuromuscular and axonal synapses of the crayfish opener muscle. J Ultrastruct Res 32:351–369
Atwood HL, Tse FW (1993) Physiological aspects of presynaptic inhibition. Adv Neural Sci 1:19–65
Atwood HL, Stevens JK, Marin L (1984) Axo-axonal synapse location and consequences for presynaptic inhibition in crustacean motor axon terminals. J Comp Neurol 225:64–74
Bailey CG, Hawkins RD, Chen MC, Kandel ER (1981) Interneurons involved in the gill-withdrawal reflex in Aplysia. IV. Morphological basis of facilitation. J Neurophysiol 45:340–360
Barron DH, Matthews BHC (1938) Dorsal root reflexes. J Physiol (Lond) 94:27–29P
Baxter DA, Bittner GD (1991) Synaptic plasticity of crayfish neuromuscular junctions: presynaptic inhibition. Synapse 7:244–251
Belardetti F, Kandel ER, Siegelbaum SA (1987) Neuronal inhibition by the peptide FMRF amide involves opening of SK+ channels. Nature 325:153–156
Bernard J (1987) Effectiveness of the cereal chordotonal inhibitory organ in the cockroach. Synaptic activity during imposed cereal movements. Comp Biochem Physiol [A] 87:53–56
Bittner GD, Baxter DA (1991) Synaptic plasticity at crayfish neuromuscular junctions: facilitation and augmentation. Synapse 7:235–243
Blagburn JM, Sattelle DB (1987) Presynaptic depolarization mediates presynaptic inhibition at a synapse between an identified mechanosensory neurone and giant interneurone 3 in the first instar cockroach, Periplaneta americana. J Exp Biol 127:135–157
Boyan GS (1988) Presynaptic inhibition of identified wind-sensitive afferents in the cereal system of the locust. J Neurosci 8:2748–2757
Brezina V, Eckert R, Erxleben C (1987) Suppression of calcium current by an endegenous neuropeptide in neurons of Aplysia californica. J Physiol 388:565–595
Bryan JS, Krasne FB (1977a) Protection from habituation of the crayfish lateral giant fibre escape response. J Physiol (Lond) 271:351–368
Bryan JS, Krasne FB (1977b) Presynaptic inhibition: the mechanism of protection from habituation of the crayfish lateral giant fibre escape response. J Physiol (Lond) 271:369–390
Burrows M (1985) The processing of mechanosensory information by spiking local interneurons in the locust. J Neurophysiol 54:463–478
Burrows M, Laurent G (1993) Synaptic potentials in the central terminals of locust proprioceptive afferents generated by other afferents from the same organ. J Neurose 13(2):808–819
Burrows M, Matheson T (1994) A presynaptic gain control mechanism among sensory neurons of a locust leg proprioceptor. J Neurosci 14(l):272–282
Büschges A, Wolf H (1995) Non-spiking local interneurons in insect leg motor control. I. Common layout and species. Specific response properties of femur-tibia joint control pathways in stick insect and locust. J Neurophysiol 73:1843–1860
Byrne JH, Kandel ER (1996) Presynaptic facilitation revisited: state and time dependance. J Neurosci 16:425–435
Cattaert D, El Manira A, Marchand A, Clarac F (1990) Central control of the sensory afferent terminals from a leg chordotonal organ in crayfish in vitro preparation. Neurosci Lett 108:81–87
Cattaert D, El Manira A, Clarac F (1992) Direct evidence for presynaptic inhibitory mechanisms in crayfish sensory afferents. J Neurophysiol 67:610–624
Cattaert D, El Manira A, Clarac F (1994a) Chloride conductance process both presynaptic inhibition and antidromic spikes in primary afferent. Brain Res 666:109–112
Cattaert D, Araque A, Buno W, Clarac F (1994b) Nicotinic and muscarinic activation of motoneurons in the crayfish locomotor network. J Neurophysiol 72:1622–1633
Chiel HJ, Kupfermann U, Weiss KR (1988) An identified histaminergic neuron can modulate the outputs of buccal-cerebral interneurons in Aplysia via presynaptic inhibition. J Neurosci 8:49–63
Chrachri A, Clarac F (1989) Synaptic connections between motor neurons and interneurons in the fourth thoracic ganglion of the crayfish, Procambarus clarkii. J Neurophysiol 62:1237–1250
Chrachri A, Clarac F (1990) Fictive locomotion in the fourth thoracic ganglion of the crayfish Procambarus clarkii. J Neurosci 10(3):707–719
Chrachri A, Williamson R (1993) Electrical coupling between primary hair cells in the statocyst of the squid, Alloteuthis sublata. Neurosci Lett 161:221–231
Clarac F, El Manira A, Cattaert D (1992) Presynaptic control as a mechanism of sensory-motor integration. Curr Opin Neurobiol 2:764–769
Coleman MJ, Nusbaum MP (1994) Functional consequences of compartimentalization of Synaptic input. J Neurosci 14(11):6544–6552
Coleman MJ, Meyrand P, Nusbaum MP (1995) A switch between two modes of synaptic transmission mediated by presynaptic inhibition. Nature 378:502–505
Daley DL, Delcomyn F (1980) Modulation of the excitability of cockroach giant interneurons during walking. II. Central and peripheral components. J Comp Physiol 138:241–251
Delcomyn F, Daley DL (1979) Central excitation of cockroach giant interneurons during walking. J Comp Physiol 130:39–48
Dudel J, Kufler SW (1961) Presynaptic inhibition at the crayfish neuromuscular junction. J Physiol (Lond) 155:543–562
Eccles JC, Magni F, Willis WD (1962) Depolarization of central terminals of group I afferent fibres from muscle. J Physiol (Lond) 160:62–93
Edwards DH (1990) Mechanisms of depolarizing inhibition at the crayfish giant motor synapse. I. Electrophysiology. J Neurophysiol 64:532–540
El Manira A, Clarac F (1991) GABA-mediated presynaptic inhibition in crayfish primary afferents by non-A, non-B GABA receptors. Eur J Neurosci 3:1208–1218
El Manira A, Clarac F (1994) Presynaptic inhibition is mediated by histamine and GABA in the crustacean escape reaction. J Neurophysiol 71:1088–1095
El Manira A, Cattaert D, Clarac F (1991) Monosynaptic connections mediate resistance reflex in crayfish walking legs. J Comp Physiol 68:337–349
El Manira A, Cattaert D, Wallen P, Di Caprio RA, Clarac F (1993) Electrical coupling of mechanoreceptor afferents in the crayfish: a possible mechanism for enhancement of sensory signal transmission. J Neurophysiol 69:2248–2251
Fisher Y, Parnas I (1996) Activation of GABA(B) receptors at individual releasing boutons of the crayfish opener neuromuscular junction produces presynaptic inhibition. J Neurophysiol 75:1377–1385
Frank K, Fuortes MG (1957) Presynaptic and postsynaptic inhibition of monosynaptic reflexes. Fed Proc 16:39–40
Fricke RA, Kennedy D (1983) Inhibition of mechanosensory neurons in the crayfish. II. Presynaptic inhibition of primary afferents by a central proprioceptive tract. J Comp Physiol [A] 153:443–453
Fricke RA, Block GD, Kennedy D (1982) Inhibition of mechanosensory neurons in the crayfish. J Comp Physiol [A] 149:251–262
Fuchs PA, Getting PA (1980) Ionic basis of presynaptic inhibitory potentials at crayfish claw opener. J Neurophysiol 43(6):1547–1557
Glantz RM, Wang-Bennett L, Waldrop B (1985) Presynaptic inhibition in the crayfish brain. I. Inhibition of a central synapse and synaptic events in presynaptic terminals. J Comp Physiol [A] 156:477–487
Gossard JP, Cabelguen JM, Rossignol S (1989) Intra-axonal recordings of cutaneous primary afferents during fictive locomotion in the cat. J Neurophysiol 5:1177–1188
Gossard JP, Cabelguen JM, Rossignol S (1991) An intracellular study of muscle primary afferents during fictive locomotion in the cat. J Neurophysiol 65:914–926
Govind CK, Atwood HL, Pearce J (1995) Inhibitory axo-axonal and neuromuscular synapses in the crayfish opener muscle: membrane definition and ultrastructure. J Comp Neurol 351:476–488
Hatsopoulos NG, Burrows M, Laurent G (1995) Hysteresis reduction in proprioception using presynaptic shunting inhibition. J Neurophysiol 73(3):1031–1042
Haydon PG, Winlow W (1982) Multipolar neurones of Lymnaea stagnalis I. Multiple spike initiation sites and propagation failure allow neuronal compartmentalization. J Comp Physiol [A] 147:503–510
Haydon PG, Man-Son-Hing H, Doyle RT, Zoran M (1991) FMRF amide modulation of secretory machinery underlying presynaptic inhibition of synaptic transmission requires a pertussis toxin-sensitive G-protein. J Neurosci 11:3851–3860
Hedwig B, Burrows M (1996) Presynaptic inhibition of sensory neurons during kicking movements in the locust. J Neurophysiol 75:1221–1232
Hodgkin AL, Horowitz P (1959) The influence of potassium and chloride ions on the membrane potential of single muscle fibres. J Physiol (Lond) 148:127–160
Hue B, Callec JJ (1983) Presynaptic inhibition in the cereal-afferent giant-interneurone synapses of the cockroach Periplaneta americana L. J Insect Physiol 29(10):741–748
Katz PS, Frost WN (1995) Intrinsic neuromodulation in the Tritonia swim CPG: the serotonergic dorsal swim interneurons act presynaptically to enhance transmitter release from interneuron C2. J Neurosci 15 (9):6035–6045
Kennedy D, Calabrese RL, Wine JJ (1974) Presynaptic inhibition: primary afferent depolarization in crayfish neurons. Science 186:451–454
Kennedy D, McVitte J, Calabrese R, Fricke RA, Craelius W, Chiapella P (1980) Inhibition of mechanosensory interneurons in the crayfish. I. Presynaptic inhibition from giant fibers. J Neurophysiol 43:1495–1509
Kirk MD (1985) Presynaptic inhibition in the crayfish CNS: pathways and synaptic mechanisms. J Neurophysiol 54:1305–1325
Kirk MD, Govind CK (1990) Presynaptic inhibition of primary afferent synapses in the crayfish. In: Wiese K, Krenz WD, Tautz J, Reichert H, Mulloney B (eds) Frontiers in crustacean Neurobiology Birkhäuser, Basel, pp 140–151
Kirk MD, Wine J (1984) Identified interneurons produce both primary afferent depolarization and presynaptic inhibition. Science 225:854–856
Krasne F, Bryan JS (1973) Habituation: regulation through presynaptic inhibition. Science 182:590–592
Kretz R, Shapiro E, Kandel ER (1986a) Presynaptic inhibition produced by an identified presynaptic inhibitory neuron. I. Physiological mechanisms. J Neurophysiol 55:113–146
Kretz R, Shapiro E, Bailey CH, Chen M, Kandel ER (1986b) Presynaptic inhibition produced by an identified inhibitory neuron. II. Presynaptic conductance changes caused by histamine. J Neurophysiol 55:131–146
Levine RB, Murphey RK (1980) Pre- and post-synaptic inhibition of identified giant interneurons in the cricket (Acheta domesticus). J Comp Physiol 135:269–282
Lyon RF (1991) Automatic gain control in cochlear mechanics. In: Dallis P, Geisler CD, Matthews JW, Ruggero MA, Steels CR (eds) The mechanics and biophysics in hearing. Springer, Berlin Heidelberg New York, pp 395–402
Mackey SL, Glanzman DL, Small SA, Dyke AM, Kandel ER, Hawkins RD (1987) Tail shock produces inhibition as well as sensitization of the siphon-withdrawal reflex of Aplysia: possible behavioral role for presynaptic inhibition mediated by the peptide Phe-Met-Arg-Phe-NH2. Proc Natl Acad Sci USA 84:8730–8734
Marchand AR, Liebrock CS (1994) Functional aspect of central electrical coupling in mechanoreceptor afferent of crayfish. Brain Res 667(1):98–106
Meyrand P, Simmers J, Moulins M (1994) Dynamic construction of a neural network from multiple pattern generators in the lobster stomatogastric nervous system. J Neurosci 14:630–644
Murphey RK, Palka J (1974) Efferent control of cricket giant fibres. Nature 248:249–251
Miwa A, Vi M, Kawai N (1990) G-protein is coupled to presynaptic glutamate and GABA receptors in lobster neuromuscular synapse. J Neurophysiol 63:173–180
Nusbaum MP, Weimann JM, Golowasch J, Marder E (1992) Presynaptic control of modulatory fibers by their neural network targets. J Neurosci 12:2706–2714
Parnas I, Grossmann Y (1973) Presynaptic inhibition in the phallic neuromuscular system of the cockroach Periplaneta americana. J Comp Physiol 82:23–32
Paul DH (1989) Nonspiking stretch receptors of the crayfish swimmeret receive an efference copy of the central motor pattern for the swimmeret. J Exp Biol 141:256–264
Pearson KG, Goodman CS (1981) Presynaptic inhibition of transmission from identified interneurons in locust central nervous system. J Neurophysiol 45:501–515
Pichon Y, Callec JJ (1970) Further studies on synaptic transmission in insects. I. External recording of synaptic potentials in a single giant axon of the cockroach Periplanata americana. J Exp Biol 52:257–265
Piomelli D, Volterra A, Dale N, Siegelbaum SA, Kandel ER, Schwartz JH, Belardetti F (1987) Lipoxygenase metabolites of arachidonic acid as second messengers for presynaptic inhibition of aplysia sensory cells. Nature 328:38–43
Plummer MR, Camhi JM (1981) Discrimination of sensory signals from noise in the escape system of the cockroach: the role of wind acceleration. J Comp Physiol 142:346–357
Quevedo J, Eguibar JR, Jimenez I, Rudomin P (1992) Differential action of (-)-baclofen on the primary afferent depolarization produced by segmental and descending inputs. Exp Brain Res 91:29–45
Rathmayer W (1990) Inhibition through neurons of the common inhibitory type (CI-neurons) in crab muscle. In: Wiese K, Krentz WD, Tautz J, Reichert H, Mulloney B (eds) Frontiers in crustacean neurobiology. Birkhäuser, Basel, pp 271–278
Rathmayer W, Maïer L (1987) Muscle fiber types in crabs: studies on single identified muscle fibers. Am Zool 27:1067–1077
Rudomin P (1990) Presynaptic inhibition of muscle spindle and tendon organ afferents in the mammalian spinal cord. Trends Neurosci 13:499–505
Rudomin P, Jimenez I, Enriquez M (1991) Effects of stimulation of Group I afferents from flexor muscles on heterosynaptic facilitation of monosynaptic reflexes produced by Ia and descending inputs: a test for presynaptic inhibition. Exp Brain Res 85:93–102
Ryall RW (1978) Presynaptic inhibition. Trends Neurosci 1:164–166
Schmidt RF (1971) Presynaptic inhibition in the vertebrate central nervous system. Ergeb Biol 63:20–101
Segev I (1990) Computer study of presynaptic inhibition controlling the spread of action potentials into axon terminals. J Neurophysiol 63:987–998
Siegelbaum SA, Camardo JS, Kandel ER (1982) Serotonin and cyclic AMP close single K+ channels in Aplysia sensory neurons. Nature 299:413–417
Sillar KT. Skorupski P (1986) Central input to primary afferent neurons in crayfish Pacifastacus leniusculus is correlated with motor output of thoracic ganglia. J Neurophysiol 55:678–688
Skorupski P, Sillar KT (1986) Phase-dependent reversal of reflexes mediated by the thoracocoxal muscle receptor organ in the crayfish Pacifasticus leniusculus. J Neurophysiol 55:689–696
Stuart GJ, Redman SJ (1992) The role of GABAA and GABAB receptors in presynaptic inhibition of Ia EPSPs in cat spinal motoneurones. J Physiol (Lond) 447:674–692
Von Holst E, Mittelstaedt H (1950) Das Reafferenzprinzip. Naturwissenschaften 37:464–476
Wallin BG (1967) The relation between external potassium concentration, membrane potential and internal ion concentrations in a crayfish axon. Acta Physiol Scand 70:431–448
Wang-Bennett LT, Glantz RM (1985) Presynaptic inhibition in the crayfish brain. II. Morphology and ultrastracture of the terminal aborization. J Comp Physiol [A] 156:605–617
Watson AHD (1992) Presynaptic modulation of sensory afferent in the invertebrate and vertebrate nervous system. Comp Biochem Physiol [A] 103 (2):227–239
Watson AHD, England RCD (1991) The distribution of and interactions between GABA-immunoreactive and non-immunoreactive processes presynaptic to Campaniform Sensilla on the trochantes of the locust leg. Cell Tissue Res 266:331–341
Watson AHD, Pflüger HJ (1994) Distribution of input synapses from processes exhibiting GABA or glutamate-like immunoreactivity onto terminals of posternal filiform afferents in the locust. J Comp Neurol 343:617–629
Watson AHD, Storm-Mathisen J, Ottersen OP (1991) GABA and glutamate-like immunoreactivity in processes presynaptic to afferents form hair plates on the proximal joints of the locust leg. J Neurocytol 20:796–809
Watson AHD, Burrows M, Leitch B (1993) GABA-immunoreactivity in processes presynaptic to the terminals of afferents from a locust leg proprioceptor. J Neurocytol 22:547–557
Weiss KR, Chiel HJ, Kupfermann (1986) Sensory function and gating of histaminergic neuron C2 in Aplysia. J Neurosci 6:2416–2426
Wiese K, Calabrese RL, Kennedy D (1976) Integration of directional mechanosensory input by crayfish interneurons. J Neurophysiol 39:834–843
Wildman MH, Cannone AJ (1991) Interaction between afferent neurones in a crab muscle receptor organ. Brain Res 565:175–178
Wine JJ (1984) The structural basis of an innate behavioral pattern. J Exp Biol 112:283–319
Wolf H, Burrows M (1995) Proprioceptive sensory neurons of a locust leg receive rhythmic presynaptic inhibition during walking. J Neurosci 15(8):5623–5636
Zucker RS (1972) Crayfish escape behavior and central synapses. I. Neural circuit exciting lateral giant fibres. J Neurophysiol 35:599–620
Zucker RS (1974) Crayfish neuromuscular facilitation activated by constant presynaptic action potentials and depolarizing pulses. J Physiol (Lond) 241:69–89
Zytnicki D, Lafleur J, Horcholle-Bossavit G, Lamy F, Jamy L (1990) Reduction of Ib autogenic inhibition in motoneurons during contractions of an ankle etensor muscle in the cat. J Neurophysiol 64:1380–1389
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Clarac, F., Cattaert, D. Invertebrate presynaptic inhibition and motor control. Exp Brain Res 112, 163–180 (1996). https://doi.org/10.1007/BF00227635
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00227635