Summary
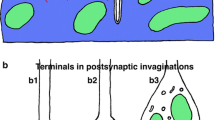
Synaptic connections were studied by means of electron microscopy in the sensory pineal organ of the ayu, Plecoglossus altivelis, a highly photosensitive teleost species. Three types of specific contacts were observed in the pineal end-vesicle: 1) symmetrically organized gap junctions between the basal processes of adjacent photoreceptor cells; 2) sensory synapses endowed with synaptic ribbons, formed by basal processes of photoreceptor cells and dendrites of pineal neurons; 3) conventional synapses between pineal neurons, containing both clear and dense-core vesicles at the presynaptic site. Based on these findings, the following interpretations are given: (i) The gap junctions may be involved in an enhancement of electric communication and signal encoding between pineal photoreceptor cells. (ii) The sensory synapses transmit photic signals from the photoreceptor cells to pineal nerve cells. (iii) The conventional synapses are assumed to be involved in a lateral interaction and/or summation of information in the sensory pineal organ. A concept of synaptic relationships among the sensory and neuronal elements in the pineal organ of the ayu is presented.
Similar content being viewed by others
References
Andrew RD, MacVicar BA, Dudek FE, Hatton GI (1981) Dye transfer through gap junctions between neuroendocrine cells of rat hypothalamus. Science 211:1187–1189
Baylor DA, Fuortes MGF, O'Bryan PM (1971) Receptive fields of cones in the retina of the turtle. J Physiol 214:265–294
Bayrhuber H (1972) Über die Synapsenformen und das Vorkommen von Acetylcholinesterase in der Epiphyse von Bombina variegata (L.), (Anura). Z Zellforsch 126:278–296
Bennett MVL, Trinkaus JP (1970) Electrical coupling between embryonic cells by way of extracellular space and specialized junctions. J Cell Biol 44:592–609
Bennett MVL, Aljure E, Nakajima Y, Pappas GD (1963) Electrotonic junctions between teleost spinal neurons: electrophysiology and ultrastructure. Science 141:262–264
Bergmann G (1971) Elektronenmikroskopische Untersuchungen am Pinealorgan von Pterophyllum scalare Cuv et Val (Cichlidae, Teleostei). Z Zellforsch 119:257–288
Bunt AH (1971) Enzymatic digestion of synaptic ribbons in amphibian retinal photoreceptors. Brain Res 25:571–577
Collin JP, Oksche A (1981) Stuctural and functional relationships in the nonmammalian pineal gland. In: Reiter RJ (ed) The pineal organ Vol I Anatomy and Biochemistry. CRC, Boca Raton, pp 27–67
Dowling JE (1968) Synaptic organization of the frog retina: an electron microscopic analysis comparing the retinas of frogs and primates. Proc R Soc Lond [Biol] 170:205–228
Ekström P, van Veen T (1983) Central connections of the pineal organ in the three-spined stickleback, Gasterosteus aculeatus L. (Teleostei). Cell Tissue Res 232:141–155
Eldred WD, Finger TE, Nolte J (1980) Central projections of the frontal organ of Rana pipiens, as demonstrated by the anterograde transport of horseradish peroxidase. Cell Tissue Res 211:215–222
Falcon J (1979) L'organe pinéal du Brochet (Esox lucius, L.) I. Etude anatomique et cytologique. Ann Biol Anim Biochem Biophys 19:445–465
Falcon J, Mocquard JP (1979) L'organe pinéal du Brochet (Esox lucius, L.) III. Voies intrapinéales de conduction des messages photosensoriels. Ann Biol Anim Biochem Biophys 19:1043–1061
Foos RY, Miyamatsu W, Yamada E (1969) Tridimensional study of an anomalous synaptic ribbon in human retina. J Ultrastruct Res 26:391–398
Friend DS, Gilula NB (1972) Variations in tight and gap junctions in mammalian tissues. J Cell Biol 53:758–776
Gilula NB, Reeves OR, Steinbach A (1972) Metabolic coupling, ionic coupling and cell contacts. Nature 235:262–265
Goodenough DA, Revel JP (1971) The permeability of isolated and in situ mouse hepatic gap junctions studied with enzymatic tracers. J Cell Biol 50:81–91
Hafeez MA, Zerihun L (1974) Studies on central projections of the pineal nerve tract in rainbow trout, Salmo gairdneri Richardson using cobalt chloride iontophoresis. Cell Tissue Res 154:485–510
Hanyu I, Niwa H (1970) Pineal photosensitivity in three teleosts, Salmo irideus, Plecoglossus altivelis and Mugil cephalus. Rev Can Biol 29:133–140
Hanyu I, Niwa H, Tamura T (1978) Salient features in photosensory function of teleostean pineal organ. Comp Biochem Physiol A61:49–54
Herwig HJ (1976) Comparative ultrastructural investigations of the pineal organ of the blind cave fish, Anoptichthys jordani, and its ancestor, the eyed river fish, Astyanax mexicanus. Cell Tissue Res 167:297–324
Herwig HJ (1979) Morphological indications for endocrine activity in the pineal organ of teleost fishes. In: Kappers JA, Pévet P (eds) The pineal gland of vertebrates including man. Prog Brain Res Vol 52, Elsevier, Amsterdam, pp 213–217
Herwig HJ (1981) The pineal organ. An ultrastructural and biochemical study on the pineal organ of Hemigrammus caudovittatus and other closely related characid fish species with special reference to the Mexican blind cave fish, Astyanax mexicanus. Thesis of Doctoral Degree, Rijksuniversity of Utrecht
Karasek M, King TS, Richardson BA, Hurlbut EC, Hansen JT, Reiter RJ (1982) Day-night differences in the number of pineal “synaptic” ribbons in two diurnal rodents, the chipmunk (Tamias striatus) and the ground squirrel (Spermophilus richardsonii). Cell Tissue Res 224:689–692
Kelly DE, Smith SW (1964) Fine structure of the pineal organ of the adult frog, Rana pipiens. J Cell Biol 22:653–674
Kemali M, Gugliemotti (1983) The connections of the frog interpeduncular nucleus (ITP) demonstrated by horseradish peroxidase (HRP). Exp Brain Res 45:349–356
King TS, Dougherty WJ (1980) Neonatal development of circadian rhythm in “synaptic” ribbon numbers in the rat pinealocyte. Am J Anat 157:335–343
Korf HW (1974) Acetylcholinesterase-positive neurons in the pineal and parapineal organs of the rainbow trout, Salmo gairdneri (with special reference to the pineal tract). Cell Tissue Res 155:475–489
Korf HW, Wagner U (1981) Nervous connections of the parietal eye in adult Lacerta s. sicula Rafinesque as demonstrated by anterograde and retrograde transport of horseradish peroxidase. Cell Tissue Res 219:567–583
Kurumado K, Mori W (1977) A morphological study of the circadian cycle of the pineal gland of the rat. Cell Tissue Res 182:565–568
Lamb TD, Simon EJ (1976) The relation between intercellular coupling and electrical noise in turtle photoreceptors. J Physiol 263:257–286
Matsushima S, Morisawa Y, Aida J, Abe K (1983) Circadian variations in pinealocytes of the Chinese hamster, Cricetulus griseus. A quantitative electron microscopic study. Cell Tissue Res 228:231–244
Matsuura T, Herwig HJ (1981) Histochemical and ultrastructural study of the nervous elements in the pineal organ of the eel, Anguilla anguilla. Cell Tissue Res 216:545–555
McNulty JA (1980) Ultrastructural observations on synaptic ribbons in the pineal organ of the goldfish. Cell Tissue Res 210:249–256
McNulty JA (1981) Synaptic ribbons in the pineal organ of the goldfish: circadian rhythmicity and the effects of constant light and constant darkness. Cell Tissue Res 215:491–497
Oguri M, Omura Y (1973) Ultrastructure and functional significance of the pineal organ of teleosts. In: Chavin W (ed) Responses of fish to environmental changes. Charles C Thomas, Springfield, pp 412–434
Ohba S, Wake K, Ueck M (1979) Histochemical and electron microscopical findings in the pineal organ of Carassius gibelio (Landsd.). In: Kappers JA, Pévet P (eds) The pineal gland of vertebrates including man. Prog Brain Res Vol 52, Elsevier, Amsterdam, pp 93–96
Oksche A (1971) Sensory and glandular elements of the pineal organ. In: Wolstenholme GEW, Knight J (eds) The pineal gland, Churchill Livingstone, Edingburgh London, pp 127–146
Oksche A, Hartwig HG (1979) Pineal sense organs-components of photoneuroendocrine systems. In: Kappers JA, Pévet P (eds) The pineal gland of vertebrates including man. Prog Brain Res Vol 52, Elsevier, Amsterdam, pp 113–130
Oksche A, Kirschstein H (1971) Weitere elektronenmikroskopische Untersuchungen am Pinealorgan von Phoxinus laevis (Teleostei, Cyprinidae). Z Zellforsch 112:572–588
Oksche A, Vaupel-von Harnack M (1963) Elektronenmikroskopische Untersuchungen an der Epiphysis cerebri von Rana esculenta L. Z Zellforsch 59:582–614
Omura Y (1975) Influence of light and darkness on the ultrastructure of the pineal organ in the blind cave fish, Astyanax mexicanus. Cell Tissue Res 160:99–112
Omura Y (1979) Light and electron microscopic studies on the pineal tract of rainbow trout, Salmo gairdneri. Rev Can Biol 38:105–118
Omura Y (1980) Histochemical and ultrastructural studies on the nervous organization of the pineal organ of the ayu, Plecoglossus altivelis. Bull Jpn Soc Sci Fish 46:1483–1488
Omura Y, Ali MA (1980) Responses of pineal photoreceptors in the brook and rainbow trout. Cell Tissue Res 208:111–122
Omura Y, Ali MA (1981) Ultrastructure of the pineal organ of the killifish, Fundulus heteroclitus, with special reference to the secretory function. Cell Tissue Res 219:355–369
Omura Y, Ali MA (1982) Effect of hypophysectomy on the synaptic ribbons in the pineal organ of the killifish Fundulus heteroclitus. Bull Jpn Soc Sci Fish 48:1679–1684
Omura Y, Oguri M (1971) The development and degeneration of the photoreceptor outer segment of the fish pineal organ. Bull Jpn Soc Sci Fish 37:851–860
Omura Y, Kitoh J, Oguri M (1969) The photoreceptor cell of the pineal organ of Ayu, Plecoglossus altivelis. Bull Jpn Soc Sci Fish 35:1067–1071
Osborne MP, Thornhill RA (1972) The effect of monoamine depleting drugs upon the synaptic bars in the inner ear of thebullfrog (Rana catesbeiana). Z Zellforsch 127:347–355
Owman C, Rüdeberg C (1970) Light, fluorescence, and electron microscopic studies on the pineal organ of the pike, Esox lucius L., with special regard to 5-hydroxytryptamine. Z Zellforsch 107:522–550
Paul E, Hartwig HG, Oksche A (1971) Neurone und zentralnervöse Verbindungen des Pinealorgans der Anuren. Z Zellforsch 112:466–493
Raviola E, Gilula NB (1973) Gap junctions between photoreceptor cells in the vertebrate retina (membranes/electron microscopy/freeze-fracturing). Proc Natl Acad Sci USA 70:1677–1681
Ribi WA (1978) Gap junctions coupling photoreceptor axons in the first optic ganglion of the fly. Cell Tissue Res 195:299–308
Rüdeberg C (1969) Light and electron microscopic studies on the pineal organ of the dogfish, Scyliorhinus canicula L. Z Zellforsch 96:548–581
Rüdeberg C (1971) Structure of the pineal organs of Anguilla anguilla L. and Lebistes reticulatus Peters (Teleostei). Z Zellforsch 122:227–243
Shiraishi Y, Takeda T (1961) The influence of photoperiodicity on the maturation of Ayu-fish, Plecoglossus altivelis. Bull Freshwater Fish Res Lab 11:69–81
Sjöstrand FS (1958) Ultrastructure of retinal rod synapses of the guinea pig eye as revealed by three dimensional reconstructions from serial sections. J Ultrastruct Res 2:122–170
Smith CA, Sjöstrand FS (1961) A synaptic structure in the hair cells of the guinea-pig cochlea. J Ultrastruct Res 5:184–192
Smith JG, Baumann F, Fuortes MGF (1965) Electrical connections between visual cells in the ommatidium of Limulus. Science 147:1446–1448
Szamier RB, Wachtel AW (1970) Special cutaneous receptor organs of fish. VI. Ampullary and tuberous organs of Hypopomus. J Ultrastruct Res 30:450–471
Takahashi H (1969) Light and electron microscopic studies on the pineal organ of the goldfish, Carassius auratus L. Bull Fac Fish Hokkaido Univ 20:143–157
Theron JJ, Biagio R, Meyer AC (1981) Circadian changes in microtubules, synaptic ribbons and synaptic ribbon fields in the pinealocytes of the baboon (Papio ursinus). Cell Tissue Res 217:405–413
Ueck M, Kobayashi H (1972) Vergleichende Untersuchungen über Acetylcholinesterase-haltige Neurone im Pinealorgan der Vögel. Z Zellforsch 129:140–160
Vigh-Teichmann I, Korf HW, Oksche A, Vigh B (1982) Opsinimmunoreactive outer segments and acetylcholinesterase-positive neurons in the pineal complex of Phoxinus phoxinus (Teleostei, Cyprinidae). Cell Tissue Res 227:351–369
Vollrath L (1973) Synaptic ribbons of a mammalian pineal gland. Circadian changes. Z Zellforsch 145:171–183
Vollrath L (1981) The pineal organ. Handb Mikrosk Anat Mensch VI/7, Springer, Berlin Heidelberg New York
Wake K (1973) Acetylcholinesterase-containing nerve cells and their distribution in the pineal organ of the goldfish, Carassius auratus. Z Zellforsch 145:287–198
Wake K, Ueck M, Oksche A (1974) Acetylcholinesterase-containing nerve cells in the pineal complex and subcommissural area of the frogs, Rana ridibunda and Rana esculenta. Cell Tissue Res 154:423–442
Witkovsky P, Dowling JE (1969) Synaptic relationships in the plexiform layers of carp retina. Z Zellforsch 100:60–82
Author information
Authors and Affiliations
Additional information
Fellow of the Alexander von Humboldt Foundation, Federal Republic of Germany
Rights and permissions
About this article
Cite this article
Omura, Y. Pattern of synaptic connections in the pineal organ of the ayu, Plecoglossus altivelis (Teleostei). Cell Tissue Res. 236, 611–617 (1984). https://doi.org/10.1007/BF00217230
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00217230