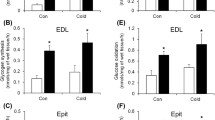
Summary
Growing rats (4 weeks old) were kept for 3 weeks at 11° C and 24° C respectively. The cold-adapted animals showed a significantly higher oxygen consumption (64%). Volume density of subsarcolemmal and interfibrillar mitochondria as well as volume density of fat droplets were estimated in M. soleus and the diaphragm of both groups. In cold-adapted animals, the total volume of mitochondria was significantly increased by 24% in diaphragm and 37% in M. soleus. The volume of subsarcolemmal mitochondria was almost doubled in each muscle, but the volume of interfibrillar mitochondria did not change significantly. The surface of the inner mitochondrial membranes per unit volume of mitochondrion in M. soleus was significantly increased both in interfibrillar and subsarcolemmal mitochondria, whereas the surface of the outer mitochondrial membranes per unit volume of mitochondrion was increased only in the subsarcolemmal mitochondria. The volume of fat droplets in the diaphragm and M. soleus of cold adapted animals increased significantly by 62% and 150% respectively.
Similar content being viewed by others
References
Ariano MA, Armstrong RB, Edgerton VR (1973) Hindlimb muscle fiber populations of five mammals. J Histochem Cytochem 21:51–55
Baldwin KM, Klinkerfuss GH, Terjung RL, Molé PA, Holloszy JO (1972) Respiratory capacity of white, red and intermediate muscle: adaptive response to exercise. Am J Physiol 222:373–379
Behrens WA, Himms-Hagen J (1977) Alteration in skeletal muscle mitochondria of cold acclimated rats: association with enhanced metabolic response to noradrenaline. J Bioenerg Biomembr 9:41–64
Benzi G, Panceri P, Di Bernardi M, Villa R, Arcelli E, d'Angelo L, Arrigoni E, Berte F (1975) Mitochondrial enzymatic adaptation of skeletal muscle to endurance training. J Appl Physiol 38:565–569
Bolender RP, Paumgartner D, Losa G, Muellener O, Weibel ER (1978) Integrated stereological and biochemical studies on hepatocytic membranes. J Cell Biol 77:565–583
Bukowiecki L, Himms-Hagen J (1971) Decreased half-life of some mitochondrial proteins in skeletal muscle and brown adipose tissue of cold-acclimated rats. Can J Physiol Pharmacol 49:1015–1018
Bukowiecki L, Himms-Hagen J (1976) Alterations of mitochondrial protein metabolism in liver, brown adipose tissue and skeletal muscle during cold acclimation. Biochim Biophys Acta 428:491–599
Burri PH, Weibel ER (1971) Morphometric estimation of pulmonary diffusion capacity. II. Effect of PO2 on the growing lung. Respir Physiol 11:247–264
Burri PH, Gehr P, Müller K, Weibel ER (1976) Adaptation of the growing lung to increased VO 2. I. IDPN as an inducer of hyperactivity. Respir Physiol 28:129–140
Bylund AC, Bjurö T, Cederblad G, Holm J, Lundholm K, Sjöström M, Aengquist KA, Scherstén T (1977) Physical training in man. Skeletal muscle metabolism in relation to muscle morphology and running ability. Eur J Appl Physiol 36:151–169
Cotter M, Hudlicka O, Pette D, Staudte H, Vrbova G (1973) Changes of capillary density and enzyme pattern in fast rabbit muscles during long-term stimulation. J Physiol 230:34–35
Desautels M, Himms-Hagen J (1980) Parallel regression of cold-induced changes in ultrastructure, composition and properties of brown adipose tissue mitochondria during recovery of rats from acclimation to cold. Can J Physiol Pharmacol 56:110–122
Foster DO, Frydman ML (1978) Nonshivering thermogenesis in the rat. II. Measurements of blood flow with microspheres point to brown adipose tissue as the dominant site of the calorigenesis induced by noradrenaline. Can J Physiol Pharmacol 56:110–122
Foster DO, Frydman ML (1979) Tissue distribution of cold-induced thermogenesis in conscious warmor cold-acclimated rats reevaluated from changes in tissue blood flow: the dominant role of brown adipose tissue in the replacement of shivering by nonshivering thermogenesis. Can J Physiol Pharmacol 57:257–270
Gehr P, Hugonnaud C, Burri PH, Bachofen H, Weibel ER (1978) Adaptation of the growing lung to increased V O 2. III. The effect of cold exposure to cold environment in rats. Respir Physiol 32:345–353
Gollnick PD, King DW (1969) Effect of exercise and training on mitochondria of rat skeletal muscle. Am J Physiol 216:1502–1509
Guernsey DL, Stevens ED (1977) The cell membrane sodium pump as a mechanism for increasing thermogenesis during cold acclimation in rats. Science 196:908–910
Himms-Hagen J (1976) Cellular thermogenesis. Ann Rev Physiol 38:315–351
Holloszy JO (1975) Adaptation of skeletal muscle to endurance training. Med Sci Sports 7:155–164
Holloszy JO, Booth FW (1976) Biochemical adaptations to endurance exercise in muscle. Ann Rev Physiol 38:273–291
Holloszy JO, Oscai LB, Don IJ, Molé PA (1970) Mitochondrial citric acid cycle and related enzymes: adaptive response to exercise. Biochem Biophys Res Commun 40:1368–1373
Hoppeier H, Lüthi P, Ciaassen H, Weibel ER, Howald H (1973) The ultrastructure of the normal human skeletal muscle. A morphometric analysis on untrained men, women and well-trained orienteers. Pfluegers Arch 344:217–232
Hoppeler H, Mathieu O, Krauer R, Ciaassen H, Armstrong RB, Weibel ER (1981) Design of the mammalian respiratory system. VI. Distribution of mitochondria and capillaries in various muscles. Respir Physiol 44:87–113
Howald H (1976) Ultrastructure and biochemical function of skeletal muscle in twins. Ann Hum Biol 3:455–462
Ismail-Beigi F, Edelman IS (1970) Mechanism of thyroid calorigenesis: role of active sodium transport. Proc Natl Acad Sci 67:1071–1078
Ivanow KP (1980) The muscle heat production after adaptation to cold. Med Biol 58:76–81
Janský L (1966) Body organ thermogenesis of the rat during exposure to cold and at maximal metabolic rate. Fed Proc 25:1297–1303
Janský L (1973) Non-shivering thermogenesis and its thermoregulatory significance. Biol Rev 48:85–132
Janský L, Hart JS (1968) Cardiac output and organ blood flow in warm- and cold-acclimated rats exposed to cold. Can J Physiol Pharmacol 46:653–659
Jansky L, Bartunkova R, Kockova J, Mejsnar J, Zeisberger E (1969) Interspecies differences in cold adaptation and nonshivering thermogenesis. Fed Proc 28:1053–1058
Mathieu O, Krauer R, Hoppeler H, Gehr P, Lindstedt SL, Mc Neill Alexander R, Taylor CR, Weibel ER (1981) Design of the mammalian respiratory system. VII. Scaling mitochondrial volume in skeletal muscle to body mass. Respir Physiol 44:113–129
Mejsnar J, Mejsnarova B (1971) Calorigenic action of noradrenaline measured in gracilis anticus muscle of cold adapted rats in vivo. Physiol Bohemoslov 20:389–390
Mejsnar J, Kolar F, Mala J (1980) Effects of noradrenaline and the blood perfusion rate on the oxygen consumption of intact and isolated muscles of cold-acclimated rats. Physiol Bohemoslov 29:151–160
Molé PA, Oscai LB, Holloszy JO (1971) Adaptation of muscle to exercise. Increase in levels of palmityl Co A synthetase, carnitine palmityltransferase, and palmityl Co A dehydrogenase and in the capacity to oxidize fatty acids. J Clin Invest 50:2323–2330
Müller W (1976) Subsarcolemmal mitochondria and capitalization of soleus muscle fibers in young rats subjected to an endurance training. Cell Tissue Res 174:367–389
Page E (1978) Quantitative ultrastructural analysis in cardiac membrane physiology. Am J Physiol 235:147–158
Page E, Mc Callister LP (1937) Quantitative electron microscopic description of heart muscle cells. Application to normal, hypertrophied and thyroxin-stimulated hearts. Am J Cardiol 31:172–181
Paumgartner D, Losa G, Weibel ER (1981) Resolution effect on the stereological estimation of surface and volume and its interpretation in term of fractional dimensions. Respir Physiol 38:565–569
Pette D, Müller W, Leisner E, Vrbova G (1976) Time dependent effects on contractile properties, fiber population, myosin light chains and enzymes of energy metabolism in intermittently and continuously stimulated fast twitch muscles of the rabbit. Pfluegers Arch 364:103–112
Seitz HS, Krone W, Wile H, Tarnowski W (1981) Rapid rise in plasma glucagon induced by acute cold exposure in man and rat. Pflügers Arch 389:115–120
Smith HE, Page E (1976) Morphometry of rat heart mitochondrial subcompartments and membranes: application to myocardial cell atrophy after hypophysectomy. J Ultrastr Res 55:310–41
Weibel ER (1979a) Stereological methods. Vol 1: Practical methods for biological morphometry. Academic Press, London New York Toronto
Weibel ER (1979b) Oxygen demand and the size of respiratory structures in mammals. In: Wood SC, Lenfant C (eds), Evolution of respiratory processes. Vol 13, Dekker M, New York Basel
Weibel ER (1980) Stereological methods. Vol 2: Theoretical foundations. Academic Press, London New York Toronto
Weiss K (1954) Adaptation of rats to cold air and effects on tissue oxygen consumptions. Am J Physiol 177:201–207
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Buser, K.S., Kopp, B., Gehr, P. et al. Effect of cold environment on skeletal muscle mitochondria in growing rats. Cell Tissue Res. 225, 427–436 (1982). https://doi.org/10.1007/BF00214693
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00214693