Abstract
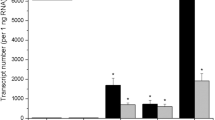
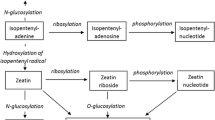
Cytokinin oxidase was extracted and partially purified from auxin- and cytokinin-dependent callus tissue of tobacco (Nicotiana tabacum L. cv. Wisconsin 38). The activity of the enzyme preparation was examined using an assay based on the conversion of tritiated N6-(Δ2-isopentenyl)adenine ([2,8-3H]iP) to adenine. Cytokinin oxidase exhibited a temperature optimum at 45–50°C and a relatively high pH optimum (8.5–9.0). The apparent Km value of the enzyme was 4.3 μM for iP. On the basis of the substrate competition assays, iP was determined to be the preferred substrate of the enzyme. Substrate competition was also observed with zeatin and the cytokinin-active urea derivative Thidiazuron. Cytokinins bearing saturated isoprenoid side chains or cyclic side chain structures, as well as auxins and abscisic acid, had no effect on the conversion of [2,8-3H]iP. The cytokinin oxidase exhibited increased activity in the presence of copper-imidazole complex in the reaction mixture. Under optimal concentrations of copper (15 mM CuCl2) and imidazole (100 mM), the enzyme activity was enhanced ca. 40-fold. Under these conditions the pH optimum was lowered to pH 6.0, whereas the temperature optimum, the apparent Km value, and the substrate specificity were not altered. Most of the enzyme moiety did not bind to the lectin concanavalin A. The characteristics of cytokinin oxidase presented here suggest that a novel molecular form of the enzyme, previously identified and characterized in Phaseolus lunatus callus cultures (Kamínek and Armstrong (1990) Plant Physiol 93:1530), also occurs in cultured tobacco tissue.
Similar content being viewed by others
Abbreviations
- Ade:
-
adenine
- iP:
-
N6-(Δ2-isopentenyl)adenine
- [2,8-3H]iP:
-
[2,8-3H]-N6-(Δ2-isopentenyl)adenine
- [9R]iP:
-
N6-(Δ2-isopentenyl)adenosine
- (diH)iP:
-
N6-isopentyladenine
- (diH)Z:
-
dihydrozeatin
- BAP:
-
N6-benzyladenine
- ( o OH)[9R]BAP:
-
N6-(o-hydroxybenzyl)adenosine
- (mOH)[9R]BAP:
-
N6-(m-hydroxybenzyl)adenosine
- IAA:
-
indole-3-acetic acid
- IBA:
-
indole-3-butyric acid
- NAA:
-
naphthalene-1-acetic acid
- ABA:
-
abscisic acid
- Con A:
-
concanavalin A
References
Abdelnour-Esquivel A, Kamínek M, Armstrong DJ (1992) Inhibition of dopamine-β-hydroxylase by cytokinins. J Plant Growth Regul 11:221–226
Armstrong DJ, Firtel RA (1989) Cytokinin oxidase activity in the cellular slime mold, Dictyostelium discoideum. Dev Biol 136:491–499
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Brownlee BG, Hall RH, Whitty CD (1975) 3-Methyl-2-butenal: An enzymatic degradation product of the cytokinin N6-(Δ2-isopentenyl)adenine. Can J Biochem 53:37–41
Burch LR, Horgan R (1989) The purification of cytokinin oxidase from Zea mays kernels. Phytochemistry 28:1313–1319
Burch LR, Horgan R (1992) Cytokinin oxidase and the degradative metabolism of cytokinins. In: Kamínek M, Mok DWS, Zazímalová E (eds) Physiology and biochemistry of cytokinins in plants. SPB Academic Publishing, The Hague, pp 29–32
Chatfield JM, Armstrong DJ (1986) Regulation of cytokinin oxidase activity in callus tissues of Phaseolus vulgaris L. cv Great Northern. Plant Physiol 80:493–499
Chatfield JM, Armstrong DJ (1987) Cytokinin oxidase from Phaseolus vulgaris callus tissues. Enhanced in vitro activity of the enzyme in the presence of copper-imidazole complexes. Plant Physiol 84:726–731
Chatfield JM, Armstrong DJ (1988) Cytokinin oxidase from Phaseolus vulgaris callus cultures. Affinity for Concanavalin A. Plant Physiol 88:245–247
Gerhäuser D, Bopp M (1990) Cytokinin oxidases in mosses. 2. Metabolism of kinetin and benzyladenine in vitro. J Plant Physiol 135:714–718
Inoue H, Hirobe M (1986) Superoxid dismutase mimetic activity of cytokinin-copper (II) complexes. Biochem Biophys Res Commun 137:372–377
Kamínek M, Armstrong DJ (1990) Genotypic variation in cytokinin oxidase from Phaseolus callus cultures. Plant Physiol 93:1530–1538
Kamínek M, Vaněk T, Motyka V (1987) Cytokinin activities of N6-benzyladenosine derivatives hydroxylated on the sidechain phenyl ring. J Plant Growth Regul 6:113–120
Laloue M, Fox JE (1985) Characterization of an imine intermediate in the degradation of isopentenyl cytokinins by a cytokinin oxidase from wheat. Abstracts of the 12th International Conference on Plant Growth Substances, Heidelberg, 1985. Abstract 03-54
Laloue M, Fox JE (1989) Cytokinin oxidase from wheat. Partial purification and general properties. Plant Physiol 90:899–906
Laloue M, Terrine C, Guern J (1977) Cytokinins: Metabolism and biological activity of N6-(Δ2-isopentenyl)adenosine and N6-(Δ2-isopentenyl)adenine in tobacco cells and callus. Plant Physiol 59:478–483
Letham DS, Palni LMS (1983) The biosynthesis and metabolism of cytokinins. Annu Rev Plant Physiol 34:163–197
McGaw BA, Horgan R (1983a) Cytokinin catabolism and cytokinin oxidase. Phytochemistry 22:1103–1105
McGaw BA, Horgan R (1983b) Cytokinin oxidase from Zea mays kernels and Vinca rosea crown-gall tissue. Planta 159:30–37
Miller CO (1985) Possible regulatory roles of cytokinins. NADH oxidation by peroxidase and a copper interaction. Plant Physiol 79:908–910
Motyka V, Kamínek M (1990) Regulation of cytokinin catabolism in tobacco callus cultures. In: Nijkamp HJJ, Van der Plas LHW, Van Aartrijk J (eds) Progress in plant cellular and molecular biology. Kluwer Academic Publishers, Dordrecht Boston London, pp 492–497
Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassays with tissue cultures. Physiol Plant 15:473–497
Pačes V, Kamínek M (1976) Effect of ribosylzeatin on the enzymatic degradation of N6-(Δ2-isopentenyl)adenosine. Nucleic Acids Res 3:2309–2314
Pačes V, Werstiuk E, Hall RH (1971) Conversion of N6-(Δ2-isopentenyl)adenosine to adenosine by enzyme activity in tobacco tissue. Plant Physiol 48:775–778
Palni LMS, Burch L, Horgan R (1988) The effect of auxin concentration on cytokinin stability and metabolism. Planta 174:231–234
Terrine C, Laloue M (1980) Kinetics of N6-(Δ2-isopentenyl)-adenosine degradation in tobacco cells. Plant Physiol 65:1090–1095
Thomas JC, Katterman FR (1986) Cytokinin activity induced by thidiazuron. Plant Physiol 81:681–683
Whitty CD, Hall RH (1974) A cytokinin oxidase in Zea mays. Can J Biochem 52:789–799
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Motyka, V., Kamínek, M. Cytokinin oxidase from auxin- and cytokinin-dependent callus cultures of tobacco (Nicotiana tabacum L.). J Plant Growth Regul 13, 1–9 (1994). https://doi.org/10.1007/BF00210700
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00210700