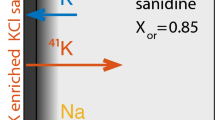
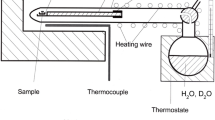
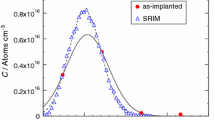
Abstract
Cation diffusion processes have been studied in single crystals of intermediate plagioclase and albite composition by tracer-diffusion experiments and optical absorption spectroscopy. Tracer-diffusivities were determined by the residual activity method, using the radioactive isotopes 22Na, 45Ca and 59Fe. In most cases, diffusion experiments were performed at 1 bar, at controlled oxygen activity and at temperatures between 750 and 1300°C. The obtained Na-diffusivities for plagioclases were much smaller then previously determined for albite. This indicates a strong composition dependence of Na-diffusion. In contrast, Ca-diffusivity in albite does not differ very much from that in intermediate plagioclases. The relative diffusivities determined for plagioclase of composition An62 at 1200° C (CO/CO2 =50∶50) were D sup*infNa ∶D sup*infFe ∶D sup*infCa =5000∶10∶1. Despite the an isotropic structure of feldspars, no difference was found for Na-and Ca-diffusion normal to (001) and normal to (010). Water pressure of 2 kbar has no influence on the Na-diffusivity. In contrast to the Ca-diffusion, a dependence on oxyggen activity was found for Na-and Fe-diffusion. Fe-diffusivity increases with decreasing oxygen activity. This can be correlated to changes in oxidation state of iron dissolved in the plagioclases. Optical absorption spectroscopy shows that iron is oxidized in the plagioclases by annealing in air. This effect can be reversed by annealing at reducing conditions. A model is proposed to explain the oxidation of iron by a chemical diffusion process in which A-vacancies are formed by out-diffusion of Na+. Preannealing of samples in air gives a temperature independent decrease of Na-diffusivity by a factor of about 2.5. This effect is explained with help of a simple disorder model for A-cations in ternary feldspars. It is concluded that Na+ diffuses via interstitials and that the A-vacancy concentration in the plagioclases is controlled extrinsically, probably by dissolved SiO2.
Similar content being viewed by others
References
Annersten H (1976) New Mössbauer data on iron in potash feldspar. Neues Jahrb Mineral Monatsh 337–343
Appleman DE, Nissen HU, Stewart DB, Clark JR, Dowty E, Huebner JS (1971) Studies of lunar plagioclases, tridymite, and cristobalite. Geochim Cosmochim Acta Suppl 2:117–133
Bardeen J, Herring C (1952) Diffusion in alloys and the Kirkendall effect. In Shockley W (ed) Imperfections in nearly perfect crystals. Wiley, New York, pp 261–288
Beaty DW, Albee AL (1980) Silica solid solution and zoning in natural plagioclase. Am Mineral 65:63–74
Behrens H (1988) Untersuchungen über Transportvorgänge in Plagioklasfeldspat (Einkristalle, Polykristalle und Gläser). Dissertation, Universität Hannover, Germany
Bell PM, Mao HK (1973) Measurements of the polarized crystal field spectra of ferrous and ferric iron in seven terristal plagio-clases. Carnegie Inst Washington Year Book 72:574–576
Beran A (1987) OH groups in nominally anhydrous framework structures: An infrared spectroscopic investigation of danburite and labradorite. Phys Chem Minerals 14:441–445
Bruno E, Facchinelli A (1974) Experimental studies on anorthite crystallization along the join CaAl2Si2O8-SiO2. Bull Soc Fr Minéral Cristallogr 97:422–432
Carmen JH, Tuttle OF (1967) Experimental verification of solid solution of excess silica in sanidine from rhyolites. Ann Meet Geol Soc Am 33
Carpenter MA, McConnell JDC (1984) Experimental delineation of the 77–01 transformation in intermediate plagioclase feldspars. Am Mineral 69:112–121
Chatterjee ND, Johannes W (1974) Thermal stability and standard thermodynamic properties of synthetic 2M1-muscovite, KAl2(AlSi3O10(OH)2). Contrib Mineral Petrol 48:89–114
Christoffersen R, Yund RA, Tullis J (1983) Inter-diffusion of K and Na in alkali feldspars: diffusion couple experiments. Am Mineral 68:1126–1133
Crank J (1975) The mathematics of diffusion, 2nd edn. Oxford University Press, London, p 414
Elphick SC, Graham CM, Dennis PR (1988) An ion microprobe study of anhydrous oxygen diffusion in anorthite: a comparison with hydrothermal data and some geological implications. Contrib Mineral Petrol 100:490–495
Foland KA (1974) Alkali diffusion in orthoclase. In: Hofmann AW, Giletti BJ, Yoder HS jr, Yund RA (eds) Geochemical transport and kinetics. Carnegie Inst, Washington 77–98
Frischat GH, Oel HJ (1966) Eine Restaktivitätsmethode zur Be-stimmung von Selbstdiffusionskoeffizienten in Festkörpern. Z Angew Phys 20:195–201
Gaite JM, Michoulier J (1970) Application de la résonance paramagnetic électronique de l'ion Fe3+ à l'étude de la structure des feldspaths. Bull Soc Fr Minéral Cristallogr 93:341–356
Giletti BJ (1984) Tracer and self-diffusion of Rb, Sr and Pb in orthoclase feldspar, a new method. Geol Soc Am Abstr Progr 16:519
Giletti BJ, Semet MP, Kasper RB (1974) Self-diffusion of potassium in low-albite using an ion microprobe. Geol Soc Am Abstr Progr 6:754
Grundy HD, Ito J (1974) The refinement of the crystal structure of a synthetic non-stoichiometric Sr feldspar. Am Mineral 59:1319–1326
Hafner SS, Virgo D, Warburton D (1971) Oxidation state of iron and plagioclase from lunar basalts. Earth Planet Sci Eett 12:159–166
Hofmeister AM, Rossman GR (1984) Determination of Fe3+ and Fe2+ concentrations in feldspar by optical absorption and EPR sepctroscopy. Phys Chem Minerals 11:213–224
Hofmeister AM, Rossman GR (1985) A model for irradiative coloration of smoky feldspar and the inhibiting influence of water. Phys Chem Minerals 12:324–332
Hokanson Sa, Yund RA (1986) Comparison of alkali interdiffusion rates for cryptoperthites. Am Mineral 71:1409–1414
Ito J (1976) High temperature solvent growth of anorthite on the join CaAl2Si2O8-SiO2. Contrib Mineral Petrol 59:187–194
Kasper RB (1975) Cation diffusion in low albite. PhD thesis, Brown University, Providence, USA
Kim K, Burley BJ (1971) Phase equilibria in the system NaAlSi3O8-NaAlSiO4-H2O with special emphasis on the stability of analcite. Can J Earth Sci 8:311–337
Kroll H, Ribbe H (1980) Determinative diagrams for Al, Si order in plagioclases. Am Mineral 65:449–457
Longhi J, Hays JF (1979) Phase equilibria and solid solution along the join CaAl2Si2O8-SiO2. Am J Sci 279:876–890
Longhi J, Walker D, Hays JF (1976) Fe and Mg in plagioclase. Geochim Cosmochim Acta Suppl 7:1281–1300
MacDonald JR (1976) Interpretation of a.c. impedance measurements in solids. In Mahan CD, Roth WL (eds) Superionic conducters. Plenum Press, New York London, pp 81–97
Marfunin AS, Bershov LV, Meilman ML, Michoulier J (1967) Paramagnetic resonance of Fe3+ in some feldspars. Schweiz Mineral Petrogr Mitt 47:13–20
McLaren AC, Marshal DB (1974) Transmission electron microscope study of the domain structures associated with the b-, c-, d-, e-and f-reflections in plagioclase feldspars. Contrib Mineral Petrol 44:237–249
Miosra NK, Venkatasubramanian VS (1977) Strontium diffusion in feldspars —a laboratory study. Geochim Cosmochim Acta 41:837–838
Muehlenbachs K, Kushiro I (1974) Oxygen isotopes exchange and equilibrium of silicates with CO2 and O2. Carnegie Inst Washington Year Book 73:232–236
Nagy KL, Giletti BJ (1986) Grain boundary diffusion of oxygen in a macroperthitic feldspar. Geochim Cosmochim Acta 50:1151–1158
Niebuhr HH, Zeira S, Hafner SS (1973) Ferric iron in plagioclase crystals from anorthosite 15415. Geochim Cosmochim Acta Suppl 4:971–982
Petrov I, Hafner SS (1988) Location of trace Fe3+ in sanidine, KAlSi3O8. Am Mineral 73:97–104
Petrovic R (1972) Alkali diffusion in alkali feldspars. PhD thesis, Yale University, New Haven, Connecticut, USA
Petrovic R (1974) Diffusion of alkali ions in alkali feldspars. In: MacKenzie WS, Zussman J (eds) The feldspars. Manchester University Press, Manchester, pp 174–182
Rainey CS, Wenk HR (1978) Intensity differences of subsidiary reflections in calcic plagioclases. Am Mineral 63:124–131
Scala CM, Hutton CR, McLaren AC (1978) NMR and EPR studies of the chemically intermediate plagioclase feldspars. Phys Chem Minerals 3:33–44
Schmalzried H (1981) Solid state reactions, 2nd edn. Verlag Chemie, Weinheim, Deerfield Beach, Basel, p 254
Schürmann K, Hafner SS (1972) On the amount of ferric iron in plagioclase from lunar igeneous rocks. Geochim Cosmochim Acta Suppl 3:615–621
Sclar CB, Kastellic RL (1979) Iron in anorthite: Synthesis and characterization of the CaAl2Si2O8-CaFeSi3O8 series. Trans Am Geophys Union EOS 60:421
Smith JV (1974) Feldspar minerals. I. Crystal structure and physical properties. Springer-Verlag, Heidelberg, p 690
Smith JV, Brown WL (1988) Feldspar Minerals. I. Crystal structures, physical, chemical, and microtextural properties, 2nd edn. Springer-Verlag, Berlin Heidelberg New York London Paris Tokyo, p 828
Smith JV, Ribbe PH (1969) Atomic movements in plagioclase feldspars: kinetic interpretation. Contrib Mineral Petrol 21:157–202
Stewart DB, Walker GW, Wright TL, Fahey JJ (1966) Physical properties of calcic labradorite from Lake County, Oregon. Am Mineral 51:177–197
Tagai T, Korekawa M (1981) Crystallographic investigation of the Huttenlocher exsolution at high temperature. Phys Chem Minerals 7:77–81
Tagai T Joswig W, Korrekawa M, Wenk HR (1980) Die Bestimmung der Al/Si-Verteilung mittels Neutronenbeugung an einem Plagioklas An 66. Z Krist 151:77–89
Tsuchiyama A, Takahashi E (1983) Melting kinetics of a plagioclase feldspar. Contrib Mineral Petrol 84:345–354
Weeks RA (1973) Paramagnetic resonance spectra of Ti3+, Fe3+, and Mn2+ in lunar plagioclases. J Geophys Res 78:2392–2401
Wegener W, Frischat GH (1982) Calculation of correlation factors by a modified Bardeen-Herring simulation method. J Phys Chem Solids 43:189–197
Weill DF, McCallum IS, Bottinga Y, Drake MJ, McKay GA (1970) Mineralogy and petrology of some Apollo 11 igenous rocks. Geochim Cosmochim Acta Suppl 1:937–955
Wenk HR, Kroll H (1984) Analysis of 78–02, 78–03 and 78–04 plagioclase structures. Bull Minéral 107:467–487
Wenk HR, Wilde WR (1973) Chemical anomalies of lunar plagioclase, described by substitution vectors and their relation to optical and structural properties. Contrib Mineral Petrol 41:89–104
Wenk HR, Joswig W, Tagai T, Korekawa M, Smith BK (1980) The average structure of An 62–66 labradorite. Am Mineral 65:81–95
Yund RA (1983) Diffusion in feldspars. In Ribbe PH (ed) Feldspar Mineralogy. Rev Mineral 2, 2nd ed, pp 203–222
Yund RA (1964) Alkali feldspar exsolution: kinetics and dependence on alkali interdiffusion. In: Brown WL (ed) Feldspar and Feldspathoids. NATO ASI series C 137, D Reidel Publ Comp, Dortrecht Boston Lancaster, pp 281–315
Yund RA, Smith BM, Tullis J (1981) Dislocation-assisted diffusion of oxygen in albite. Phys Chem Minerals 7:185–189
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Behrens, H., Johannes, W. & Schmalzried, H. On the mechanisms of cation diffusion processes in ternary feldspars. Phys Chem Minerals 17, 62–78 (1990). https://doi.org/10.1007/BF00209227
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF00209227