Summary
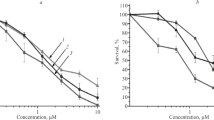
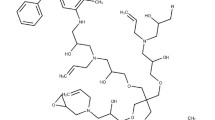
Monoclonal antibodies (IgG1) against high molecular weight antigen A-1-43 on human melanoma cell line A-375 were successfully linked to the anti-tumour protein neocarzinostatin (NCS) using the heterobifunctional reagent N-succinimidyl 3-(2-pyridyldithio)-propionate (SPDP). The conjugate retained both the reactivity of the antibody and the toxicity of the drug. The antigen-bearing cell line A-375, antigen-lacking cell line MeWo and normal skin fibroblasts were exposed to NCS-monoclonal antibody conjugates. As negative control, cells were also treated with free NCS and NCS coupled to normal mouse IgG1 antibodies. Inhibition of 3H-thymidine uptake after treatment was used to measure the biological activity of the cytotoxic drug complex or substance, respectively.
Comparing the inhibition dose for 50% uptake (ID50) it was found that the monoclonal antibody-drug complex is about 100 times more toxic for the antigen-bearing cell line than free NCS or normal mouse IgG1-NCS. This high toxicity is due to a local increase of drug concentration on these cells. With the two cell lines lacking the appropriate antigen no significant differences in the ID50 values were observed. A selectivity factor of 40–50 was obtained by comparing the cytotoxic effect of the monoclonal antibody-NCS conjugate upon the antigen-bearing as opposed to the antigen-lacking cell type. These data demonstrate, that the toxicity of NCS can be directed by monoclonal antibodies to human tumour cells carrying the corresponding surface antigen.
Similar content being viewed by others
References
Baldwin RW (1983) Monoclonal antibodies for drug-targeting in cancer therapy. Pharmacy International 4:137
Blythman HE, Casellas P, Gros O, Gros P, Jansen FK, Paolucci F, Pau B, Vidal, H (1981) Immunotoxins: Hybrid molecules of monoclonal antibodies and a toxin subunit specificially kill tumour cells. Nature 290:145
Brüggen J, Sorg C (1983) Detection of phenotypic differences on human malignant melanoma lines and their variant sublines with monoclonal antibodies. Cancer Immunol Immunother 15:200
Brüggen J, Bröcker E-B, Suter L, Redmann K, Sorg C (1984) The expression of tumour-associated antigens in primary and metastatic human malignant melanoma. Behring Inst Mitt 74:19
Brüggen J, Bröcker E-B, Suter L, Redmann K, Sorg C (1984) Cellular and malignant parameters of tumour progression in human malignant melanoma. In: Ruiter DJ, Welvaart K, Ferrone S (eds) Cutaneous melanoma and precursor lesions. Martinus Nijhoff Publish, Boston, The Haag, p 38
Carlsson J, Drevin H, Axen R (1978) Protein thiolation and reversible protein-protein conjugation. Biochem J 173:723
Collier RJ, Kaplan DA (1984) Immunotoxins. Sci Am 251:44
Ehrlich P (1913) Chemotherapy. In: Himmelweit F (ed) (1960) The collected papers of Paul Ehrlich. Pergamon press, London, vol 3, p 510
Ey PL, Prowse SJ, Jenkin CR (1978) Isolation of pure IgG1, IgG2a and IgG2b immunoglobulins from mouse serum using protein A-Sepharose. Immunochem 15:429
Gallego J, Price MR, Baldwin RW (1984) Preparation of four daunomycin-monoclonal antibody 791T/36 conjugates with anti-tumour activity. Int J Cancer 33:737
Garnett MC, Embleton MJ, Jacobs E, Baldwin RW (1983) Preparation and properties of a drug-carrier-antibody conjugate showing selective antibody-directed cytotoxicity in vitro. Int J Cancer 31:661
Gregoriadis G (1982) Use of monoclonal antibodies and liposomes to improve drug delivery. Present status and future implications. Drugs 24:261
Ishida R, Nishimoto T, Takahashi T (1979) DNA strand scission by neocarzinostatin and its relation to the inhibition of cell cycle traverse and DNA synthesis. Cell Structure and Function 4:235
Jung G, Lewis RS, Köhnlein W (1980) The generation of preneocarzinostatin and antagonism of neocarzinostatin-induced DNA strand scission in vitro. Biochim Biophys Acta 608:147
Jung G, Köhnlein W (1981) Neocarzinostatin: Controlled release of chromophore and its interaction with DNA. Biochem Biophys Res Commun 98:176
Jung G, Köhnlein W, Lüders G (1981) Biological activity of the anti-tumour protein neocarzinostatin coupled to a monoclonal antibody by N-succinimidyl 3-(2-pyridyldithio)propionate. Biochem Biophys Res Commun 101:599
Kappen LS, Goldberg IH (1980) Stabilization of neocarzinostatin nonprotein chromophore activity by interaction with apoprotein and with HeLa cells. Biochemistry 19:4786
Khokhlov AS, Cherches BZ, Reshetov PD, Smirnowa GM, Sorohina JB, Koloditskaya TA, Smirnov VV, Navashin SM, Fomina JP (1969) Physico-chemical and biological studies on actinoxanthin, an antibiotic from Actinomyces globisporus. J Antibiot 22:541
Kimura I, Ohnoshi T, Tsubota T, Sato Y, Kobayashi T, Abe S (1980) Production of tumor antibody-neocarzinostatin (NCS) conjugate and its biological activities. Cancer Immunol Immunother 7:235
Köhler G, Milstein C (1975) Continuous cultures of fused cells secreting antibody of predefined specificity. Nature 256:495
Köhnlein W, Jung G (1982) Neocarzinostatin: Molekulare Wirkungsweise und Perspektiven für die klinische Anwendung. Arzneimittel Forsch 32:474
Lewis RS, Jung G, Köhnlein W (1980) A model for the activation and inactivation of neocarzinostatin, an antitumour protein. Biochim Biophys Acta 608:138
Lippman MM, Laster WR, Abbott BJ, Venditti J, Baratta M (1975) Anti-tumour activity of macromomycin B (NCS 170105) against murine leukemias, melanoma, and lung carcinoma. Cancer Res 35:939
Maeda H (1981) Neocarzinostatin in cancer chemotherapy (Review). Anticancer Res 1:175
Manabe Y, Tsubota T, Haruta Y, Kataoka K, Okazaki M, Haisa S, Nakamura K, Kimura I (1984) Production of a macromomycin (MCR)-monoclonal antibody conjugate and its biological activity. Biochem Pharmacol 33:2143
Napier MA, Holmquist B, Strydom DJ, Goldberg IH (1979) Neocarzinostatin: Spectral characterization and separation of a non-protein chromophore. Biochem Biophys Res Commun 89:635
Pimm MV, Jones JA, Price MR, Middle JG, Embleton MJ, Baldwin RW (1982) Tumour localization of monoclonal antibody against a rat mammary carcinoma and suppression of tumour growth with adriamycin-antibody conjugates. Cancer Immunol Immunother 12:125
Povirk LF, Goldberg IH (1980) Binding of the non-protein chromophore of neocarzinostatin to deoxyribonucleic acid. Biochemistry 19:4773
Povirk LF, Dattagupta N, Warf BC, Goldberg IH (1981) Neocarzinostatin chromophore binds to deoxyribonucleic acid by intercalation. Biochemistry 20:4007
Suter L, Brüggen J, Sorg C (1980) Use of an enzyme-linked immunosorbent assay (ELISA) for screening of hybridoma antibodies against cell surface antigens. J Immunol Methods 39:407
Suter L, Bröcker E-B, Brüggen J, Ruiter DJ, Sorg C (1983) Heterogeneity of primary and metastatic human malignant melanoma as detected with monoclonal antibodies in cryostat sections of biopsies. Cancer Immunol Immunother 16:53
Thorpe PE, Edwards DC, Davies AJS, Ross WCJ (1982) Monoclonal antibody-toxin conjugates: aiming the magic bullets. In: McMichael AJ, Fabre JW (eds) Monoclonal antibodies in clinical medicine. Academic press, London, p 167
Yamashita T, Naoi N, Hidaka T, Watanabe K, Kumada Y, Takeuchi T, Umezawa H (1978) Studies on auromomycin. J Antibiot 32:330
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Lüders, G., Köhnlein, W., Sorg, C. et al. Selective toxicity of neocarzinostatin-monoclonal antibody conjugates to the antigen-bearing human melanoma cell line in vitro. Cancer Immunol Immunother 20, 85–90 (1985). https://doi.org/10.1007/BF00199779
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00199779