Summary
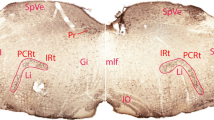
The central nucleus of the amygdala has been shown to be involved in cardiovascular regulation and the integration of arousal. In this study, the afferent input was investigated in cat by microinjecting horseradish peroxidase (HRP) into the central nucleus and examining retrogradely-labelled cells in the brain. Retrograde labelling was found in the cortex next to the sulcus ectosylvius anterior, fissura lateralis Sylvii, sulcus rhinicus anterior and posterior, sulcus suprasylvius, and pyriform and entorhinal cortices as well as in the insula and claustrum. Each of the sub-nuclei of the amygdaloid complex exhibited retrogradely-labelled perikarya. Labelled cells were also found in the diagonal band of Broca, nucl. lateralis septi, and nucl. proprius striae terminalis (bed nucl. of stria terminalis). In the hypothalamus the area preoptica medialis and lateralis, nucl. dorsomedialis, paraventricularis, periventricularis, arcuatus and mammilaris medialis were labelled. The nucl. subthalamicus, zona incerta, peripeduncular system, substantia nigra, and nucl. interpeduncularis contained HRP-marked cells. In the thalamus labelled cells were observed in the nucl. reuniens, nucl. centroposterior lateralis, nucl. latero-posterior, nucl. posterior, nucl. centro-anterior, antero-dorsalis, antero-medialis, antero-lateralis, centrum mdianum, nucl. reticularis, nucl. rhomboideus, nucl. parafascicularis and subfascicularis. The area tegmentalis Tsai and the corpora geniculata also contained labelled cells. In the brain stem, HRP-marked cells could be detected in the brachium colliculi inferioris, aqueductal grey matter, locus coeruleus, nucl. parabrachialis, in various nuclei of the formatio reticularis, in the nucl. retrofascialis, nucl. solitarius, nucl. commissuralis, nucl. ambiguus and nucl. dorsalis n. vagi. The results were compared to other neuroanatomical studies and to functional studies of the amygdala.
Similar content being viewed by others
References
Aggleton JP, Burton MJ, Passingham RE (1980) Cortical and subcortical afferents to the amygdala of the rhesus monkey (Macaca mulatta). Brain Res 190:347–368
Aghajanian GK, Wang RY (1977) Habenular and other midbrain raphe afferents demonstrated by a modified retrograde tracing technique. Brain Res 122:229–242
Amaral DG, Veazey RB, Cowan WM (1982) Some observations on hypothalamo-amygdaloid connections in the monkey. Brain Res 252:13–27
Azmitia EC (1978) The serotin-producing neurons of the midbrain median and dorsal raphe nuclei. Handbook of Psychopharmacol 9:233–314
Azmitia EC, Siegel M (1978) An autoradiographic analysis of the differential ascending projections of the dorsal and median raphe nuclei in the rat. J Comp Neurol 179:641–668
Berman AL (1968) The brain stem of the cat. In: A cytoarchitectonic atlas with stereotaxic coordinates. Madison, University of Wisconsin Press
Brushart TM, Mesulam MM (1980) Transganglionic demonstration of central sensory projections from skin and muscle with HRP-lectin conjugates. Neurosci Lett 17:1–6
Bunney BS, Aghajanian GK (1976) The precise localization of nigral afferents in the rat as determined by a retrograde tracing technique. Brain Res 117:423–435
Cassell MD, Gray TS, Kiss JZ (1986) Neuronal architecture in the rat central nucleus of the amygdala: A cytological, hodological, and immunocytochemical study. J Comp Neurol 246:478–499
Cechetto DF, Ciriello J, Calaresu FR (1983) Afferent connections to cardiovascular sites in the amygdala: A horseradish peroxidase study in the cat. J Auton Nerv Syst 8:97–110
Cedarbaum JM, Aghajanian GK (1978) Afferent projections to the rat locus coeruleus as determined by a retrograde tracing technique. J Comp Neurol 178:1–16
Conrad LCA, Pfaff DW (1976) Efferents from medial and basal fore-brain and hypothalamus in the rat. II. An autoradiographic study of the anterior hypothalamus. J Comp Neurol 169:221–262
Cranford JL, Ladner SJ, Campbell CBG, Neff WD (1978) Efferent projections of the insular and temporal neocortex in the cat. Brain Res 117:195–210
Eismann A, Knuepfer M, Stumpf H, Stock G (1982) Amygdaloid unit activity during arousal. Pflügers Arch 394:53R
Emson PC, Jessell T, Paxinos G, Cuello AC (1978) Substance P in the amygdaloid complex, bed nucleus, and stria terminalis of the rat brain. Brain Res 149:97–105
Fulwiler CE, Saper CB (1984) Subnuclear organization of the efferent connections of the parabrachial nucleus in the rat. Brain Res Rev 7:229–259
Van Hoesen GW (1981) The differential distribution, diversity and sprouting of cortical projections to the amygdala in the rhesus monkey. In: Ben-Ari Y (ed) The amygdaloid complex. Elsevier, North-Holland, pp 77–90
Hopkins DA (1975) Amygdalotegmental projections in the rat, cat, and rhesus monkey. Neurosci Lett 1:263–270
Hopkins DA, Holstege G (1978) Amygdaloid projections to the mesencephalon, pons, and medulla oblongata in the cat. Exp Brain Res 32:529–547
Hosoya Y, Matsushita M (1980) Cells of origin of the descending afferents to the lateral hypothalamic area in the rat, studied with the horseradish peroxidase method. Neurosci Lett 18:231–236
Imai H, Steindler DA, Kitai ST (1986) The organization of divergent axonal projections from the midbrain raphe nuclei in the rat. J Comp Neurol 243:363–380
Jones BE, Moore RY (1977) Ascending projections of the locus coeruleus in the rat. II. Autoradiographic study. Brain Res 127:23–53
Jones EG, Burton H, Saper CB, Swanson LW (1976) Midbrain, diencephalic and cortical relationships of the basal nucleus of Meynert and associated structures in primates. J Comp Neurol 167:385–420
Kapp BS, Schwaber JS, Driscoll PA (1985a) Frontal cortex projections to the amygdaloid central nucleus in the rabbit. Neuroscience 15:327–346 (1985a)
Kapp BS, Schwaber JS, Driscoll PA (1985b) The organization of insular cortex projections to the amygdaloid central nucleus and autonomic regulatory nuclei of the dorsal medulla. Brain Res 360:355–360
Kita H, Oomura Y (1982) An HRP-study of the afferent connections to the rat lateral hypothalamic region. Brain Res Bull 8:63–71
Kohno J, Shiosaka S, Shinoda K, Inagaki S, Tohyama M (1984) Two distinct strio-nigral substance P pathways in the rat: An experimental immunohistochemical study. Brain Res 308:309–317
Kooy D v.d., Koda LY, McGiuty JF, Gefreu CR, Bloom FE (1984) The organization of projections from the cortex, amygdala, and hypothalamus to the nucleus of the solitary tract in the rat. J Comp Neurol 224:1–24
Krettek JE, Price JL (1978a) Amygdaloid projections to subcortical structures within the basal fore-brain and brain stem in the rat and cat. J Comp Neurol 178:225–253
Krettek JE, Price JL (1978b) A description of the amygdaloid complex in the rat and cat with observations on intra-amygdaloid axonal connections. J Comp Neurol 178:255–280
Leichnetz GR, Povlishock JT, Astruc J (1976) A prefronto-amygdaloid projection in the monkey: light and electron microscopic evidence. Neurosci Lett 2:261–265
Luiton PGM, Onto T, Nishijo H, Fukuda M (1983) Differential input from the amygdaloid body to the ventromedial hypothalamic nucleus in the rat. Neurosci Lett 35:253–258
Mason ST, Fibiger HC (1979) Regional topography within noradrenergic locus coeruleus as revealed by retrograde transport of horseradish peroxidase. J Comp Neurol 187:703–724
Mesulam MM (1982) Principles of horseradish peroxidase. Neurohistochemistry and their applications for tracing neural pathways — axonal transport, enzyme histochemistry, and light microscopic analysis. In: Mesulam MM (ed) Tracing neural connections with horseradish peroxidase. John Wiley and Sons, Chichester, pp 3–135
Moga MM, Grey TS (1985) Evidence for corticotropin-releasing factor, neurotensin and somatostatin in the neural pathway from the central nucleus of the amygdala to the parabrachial nucleus. J Comp Neurol 241:275–284
Nitecka L, Amerski L, Narkiewicz O (1981) Interamygdaloid connections in the rat studied by the horseradish peroxidase method. Neurosci Lett 26:1–4
Nomura S, Mizuno N, Itoh K, Matsuda K, Sugimoto T, Nakamura Y (1979) Localization of parabrachial nucleus neurons projecting to the thalamus or the amygdala in the cat using horseradish peroxidase. Exp Neurol 64:375–385
Oldfield BJ, Silverman AJ (1985) A light microscopic HRP-study of limbic projections to the Vasopessin-containing nuclear groups of the hypothalamus. Brain Res Bull 14:143–157
DeOlmos J, Alheid GF, Beltramino CA (1985) Amygdala. In: Paxinos G (ed) The rat nervous system. Academic Press, Sydney, pp 223–334
Ottersen OP (1980) Afferent connections to the amygdaloid complex of the rat and cat. II. Afferents from the hypothalamus and the basal telencephalon. J Comp Neurol 194:267–289
Ottersen OP (1981) Afferent connections to the amygdaloid complex of the rat and cat. III. Afferents from the lower brain stem. J Comp Neurol 202:335–356
Ottersen OP (1982) Connections of the amygdala of the rat. IV. Corticoamygdaloid and intraamygdaloid connections as studied with axonal transport of horseradish peroxidase. J Comp Neurol 205:30–48
Ottersen OP, Ben-Ari Y (1979) Afferent connections to the amygdaloid complex of the rat and cat. I. Projections from the thalamus. J Comp Neurol 187:401–424
Phillipson OT (1979) Afferent projections to the ventral tegmental area of Tsai and intrafascicular nucleus: a horseradish peroxidase study in the rat. J Comp Neurol 187:117–143
Pickel VM, Segal M, Bloom FE (1974) A radioautographic study of the afferent pathways of the nucleus locus coeruleus. J Comp Neurol 155:15–42
Post S, Mai JK (1978) Evidence for amygdaloid projections to the contralateral hypothalamus and the ipsilateral midbrain in the rat. Cell Tissue Res 191:183–186
Price JL, Amaral DG (1981) An autoradiographic study of the projections of the central nucleus of the monkey amygdala. Neuroscience 1:1242–1259
Reich H, Ruprecht U, Stumpf H, Stock G (1983) Modulation of unit activity in the amygdala of unrestrained cats during the sleep-waking cycle. Neurosci Lett 35:209–214
Reinoso-Suarez F (1961) Topographischer Hirnatlas der Katze für experimentalphysiologische Untersuchungen. E. Merck, Darmstadt
Ricardo JA, Koh ET (1978) Anatomical evidence of direct projections from the nucleus of the solidarity tract to the hypothalamus, amygdala, and other forebrain structures in the rat. Brain Res 153:1–26
Ross CA, Ruggiero DA, Reis DJ (1981) Afferent projections to cardiovascular portions of the nucleus tractus solitarius in the rat. Brain Res 223:402–408
Russchen FT (1982a) Amygdalopetal projections in the cat. I. Cortical afferent connections. A study with retrograde and anterograde tracing techniques. J Comp Neurol 206:159–179
Russchen FT (1982b) Amygdalopetal projections in the cat. II. Subcortical afferent connections. A study with retrograde tracing techniques. J Comp Neurol 207:157–176
Russchen FT, Amaral DG, Price JL (1985) The afferent connections of the substantia innominata in the monkey, Macaca fascicularis. J Comp Neurol 242:1–27
Sagal M, Pickel V, Bloom F (1973) The projections of the nucleus locus coeruleus: An autoradiographic study. Life Sci 13:817–821
Saper CB (1982) Convergence of autonomic and limbic connections in the insular cortex of the rat. J Comp Neurol 210:163–173
Saper CB, Swanson LW, Cowan WM (1976) The afferent connections of the ventromedial nucleus of the hypothalamus of the rat. J Comp Neurol 169:409–442
Saper CB, Swanson LW, Cowan WM (1979) Some afferent connections of the rostral hypothalamus in the squirrel monkey (Saimiri sciureus) and cat. J Comp Neurol 184:205–242
Saper CB, Loewy AD (1980) Efferent connections of the parabrachial nucleus in rat. Brain Res 197:291–317
Schütze I, Knuepfer M, Eismann A, Stumpf H, Stock G (1987) Sensory input to single neurons in the amygdala of the cat. Exp Neurol 97:499–515
Schwaber JS, Kapp BS, Higgins G (1980) The origin and extent of direct amygdala projections to the region of the dorsal motor nucleus of the vagus and the nucleus of the solitary tract. Neurosci Lett 20:15–20
Shiosaka S, Tohyama M, Takagi H, Takahashi Y, Saitoh Y, Sakimoto T, Nakagawa H, Shimizu N (1980) Ascending and descending components of the medial forebrain bundle in the rat as demonstrated by the horseradish peroxidase-blue reaction. I. Forebrain and upper brain stem. Exp Brain Res 39:377–388
Stock G, Schlör KH, Heidt H, Buss J (1978) Psychomotor behaviour and cardiovascular patterns during stimulation of the amygdala. Pflügers Arch 376:177–184
Stock G, Stumpf H, Schlör KH (1979) Absence of sympathetic cholinergic vasodilatation in cats during early stages of affective behaviour elicited by stimulation of central amygdala, posterolateral hypothalamus and locus coeruleus. Clin Sci 57:205–208
Stock G, Ruprecht U, Stumpf H, Schlör KH (1981) Cardiovascular changes during arousal elicited by stimulation of amygdala, hypothalamus and locus coeruleus. J Auton Nerv System 3:503–510
Swanson LW, Cowan WM (1979) The connections of the septal region in the rat. J Comp Neurol 186:621–656
Takeuchi Y, McLean JH, Hopkins DA (1982) Reciprocal connections between the amygdala and parabrachial nuclei: Ultrastructural demonstration by degeneration and axonal transport of horseradish peroxidase in the cat. Brain Res 239:583–588
Turner BH, Mishkin M, Knapp M (1980) Organization of the amygdalopetal projections from modality-specific cortical association areas in the monkey. J Comp Neurol 191:515–543
Turner BH, Herkenham M (1981) An autoradiographic study of thalamo-amygdaloid connections in the rat. Anat Res 199:260A
Veening JG (1978a) Cortical afferents of the amygdaloid complex in the rat. An HRP study. Neurosci Lett 8:191–195
Veening JG (1978b) Subcortical afferents of the amygdaloid complex in the rat: An HRP-study. Neurosci Lett 8:197–202
Wakefield C, Hall E (1974) Hypothalamic projections to the amygdala in the cat. A light and electron microscopic study. Cell Tissue Res 151:499–508
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Volz, H.P., Rehbein, G., Triepel, J. et al. Afferent connections of the nucleus centralis amygdalae. Anat Embryol 181, 177–194 (1990). https://doi.org/10.1007/BF00198957
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00198957